Record Number
1347
PROSEA Handbook Number
12(2): Medicinal and poisonous plants 2
Taxon
Trachyspermum ammi (L.) Sprague ex Turrill
Protologue
Kew Bull. 7: 228 (1929).
Family
UMBELLIFERAE
Chromosome Numbers
2n = (14, 16), 18
Synonyms
Sison ammi L. (1753), Trachyspermum copticum (L.) Link (1821), Carum copticum Hiern (1871).
Vernacular Names
(True) bishop's weed, carum (En). Indonesia: mungsi (Javanese, Malay), mose (Madurese), musi (Balinese). Malaysia: jemuju, hajimuju, mungsi (Peninsular). Philippines: damoro (Tagalog, Pampangan), lamudio (Tagalog, Bikol). Thailand: phakchee (northern).
Origin and Geographic Distribution
Trachyspermum ammi is probably a native of Ethiopia and Egypt, and occurs semi-naturally in southern Europe, but is found cultivated in northern Africa, Ethiopia, western Asia, India, Iran and the former USSR, as well as here and there in South-East Asia, e.g. in Java (Indonesia), Peninsular Malaysia and the Philippines.
Uses
In South-East Asia, Trachyspermum ammi is primarily used as a medicinal plant, and to a lesser extent as a spice. The fruits are aromatic and have a pungent taste. They are considered carminative, antispasmodic and stimulant and are applied in decoction or plasters. They are also used for stomach and liver problems, a sore throat, cough, rheumatism and asthma. The essential oil is considered strongly antiseptic and is used for internal parasites. In Java, the fruits are used in mixtures drunk by women after childbirth and to induce menstruation. A decoction of the dried and crushed fruits is also used for intestinal problems and diarrhoea. In the Philippines, the fresh fruits are chewed for flatulence. In India, the essential oil is known as 'ajowan oil', and enters into the Indian Pharmacopoeia. In Ethiopia, the fruits and roots, mixed with other spices, are used against stomach problems. The fruits are taken as a vermifuge and abortifacient. In Somalia, the fruits are chewed against diarrhoea.
Trachyspermum ammi is widely cultivated as a spice for curries in India, the Mediterranean and Ethiopia. Usually the fruits are dried, roasted and ground before use. The fruits are also used in pickles, biscuits and sweets. The essential oil is sometimes added to food as a preservative.
The fruit cake left after steam distillation is rich in protein and fat and is used as cattle feed.
Trachyspermum roxburghianum (DC.) H. Wolff is cultivated on a small-scale for similar culinary and medicinal purposes as Trachyspermum ammi.
Trachyspermum ammi is widely cultivated as a spice for curries in India, the Mediterranean and Ethiopia. Usually the fruits are dried, roasted and ground before use. The fruits are also used in pickles, biscuits and sweets. The essential oil is sometimes added to food as a preservative.
The fruit cake left after steam distillation is rich in protein and fat and is used as cattle feed.
Trachyspermum roxburghianum (DC.) H. Wolff is cultivated on a small-scale for similar culinary and medicinal purposes as Trachyspermum ammi.
Production and International Trade
Small-scale cultivation is widespread throughout the distribution area of Trachyspermum ammi. In South-East Asia, Trachyspermum ammi is grown on a small scale, in home gardens, in pots, and in small, upland fields. Large-scale cultivation occurs mainly in India, and the fruits are exported to the United Kingdom, Germany, the United States, Japan and South-East Asia. The quantity of Trachyspermum ammi fruits exported from India varied from 2.4B-7 t/year between 1966—1973, but recent statistics are not available.
Properties
Depending on the origin and cultivar, the content of essential oil in Trachyspermum ammi fruits ranges from 3—10%. The main active constituents of the oil are phenols, mainly thymol (35—60%), which has strong antiseptic properties, and its isomer carvacrol. After extraction of the thymol, the residual mixture, which is called thymene, is used to perfume soap. Thymene contains terpenes like p-cymene, g-terpinene, 'ALFA'- and 'BETA'-pinene. The lipids in the fruits were extracted using a chloroform/methanol mixture. The lipid fraction consisted of about 1% hydrocarbons, 2% wax esters, 2% sterol esters, 54% triglycerides, 6% fatty acids, 6% 1,2-diglycerides, 7% glycolipids, 4% 2-monoglycerides, 7.5% 1-monoglycerides, 1.5% phosphatidyl-ethanolamines, 1% phosphatidylcholines, 0.5% lysophosphatidylethanolamines and 1% phosphatidylinositols.
The essential oil shows strong antibacterial activity against many gram-positive and gram-negative bacteria. It also showed long-term fungitoxic activity against storage fungi like Aspergillus flavus, Aspergillus niger, Claviceps oryzae-sativae and Helminthosporium oryzae, and soil-borne fungi like Macrophomina phaseolina, Pythium aphanidermatum and Rhizoctonia solani. Thymol also showed significant dose-dependent molluscicidal effects in Indoplanorbis exustus and Lymnea acuminata, and nematicidal activity against Cephalobus litoralis and Meloidogyne incognita. Furthermore, the mosquito larvicidal property of the essential oil was tested against 4th-instar larvae of Aedes aegypti, Anopheles stephensi and Culex fatigans (synonym Culex quinquefasciatus) at 0.01%, 0.1% and 1.0% concentrations, and showed high mortality after 24 hours. The essential oil is also a strong inhibitor for sprouting of potatoes stored at room temperature.
In addition, the essential oil has a parasympathomimetic effect and produces relaxation of the isolated ileum, tracheal chain and bronchial musculature in guinea-pigs. In anaesthetized rats, thymol (1—10 mg/kg), administered intravenously, produced dose-dependent reduction in blood pressure and heart rate. These effects were not blocked by atropine (1 mg/kg). In the rabbit aorta, thymol caused relaxation of norepinephrine- and potassium-induced contractions. Moreover, atropine, propranolol, indomethacin and glibenclamide did not alter the vasorelaxation caused by thymol. The results suggest that thymol is a calcium channel blocker which may explain the hypotensive and bradycardiac effects observed.
Other pharmacological effects of Trachyspermum ammi extracts include inhibition of the platelet aggregation induced by arachidonic acid, epinephrine and collagen by an ether extract of the fruits.
Aqueous and 90% ethanolic extracts were studied for their anti-reproductive potential in rats, orally dosed for 10 days after insemination, and were found to have moderate teratologic potential. The aqueous extract of the fruits was tested for anti-oxidant properties in human erythrocyte membranes against lipid peroxidation induced by FeSO4-ascorbate, and was found to exhibit 75% inhibition. It was also found to inhibit the formation of diene, triene and tetraene conjugates in human erythrocyte membrane. In addition, the essential oil also showed excellent anti-oxidant effects of stored sunflower and soya bean oil.
An aqueous extract of the fruits was evaluated for the effects on gastric acid secretion in anaesthetized rats. The stomach of anaesthetized rats was perfused at 0.15 ml/min with the extract or with acetylcholine (1 µg/ml or 10 µg/ml). Acute gastric mucosal injury was induced by leaving aspirin, at 125 mg/kg, in the stomach for 2 h before perfusion. The extract increased acid secretion, while atropine abolished the acid secretion induced by acetylcholine and significantly reduced acid induction by the extract.
The essential oil shows strong antibacterial activity against many gram-positive and gram-negative bacteria. It also showed long-term fungitoxic activity against storage fungi like Aspergillus flavus, Aspergillus niger, Claviceps oryzae-sativae and Helminthosporium oryzae, and soil-borne fungi like Macrophomina phaseolina, Pythium aphanidermatum and Rhizoctonia solani. Thymol also showed significant dose-dependent molluscicidal effects in Indoplanorbis exustus and Lymnea acuminata, and nematicidal activity against Cephalobus litoralis and Meloidogyne incognita. Furthermore, the mosquito larvicidal property of the essential oil was tested against 4th-instar larvae of Aedes aegypti, Anopheles stephensi and Culex fatigans (synonym Culex quinquefasciatus) at 0.01%, 0.1% and 1.0% concentrations, and showed high mortality after 24 hours. The essential oil is also a strong inhibitor for sprouting of potatoes stored at room temperature.
In addition, the essential oil has a parasympathomimetic effect and produces relaxation of the isolated ileum, tracheal chain and bronchial musculature in guinea-pigs. In anaesthetized rats, thymol (1—10 mg/kg), administered intravenously, produced dose-dependent reduction in blood pressure and heart rate. These effects were not blocked by atropine (1 mg/kg). In the rabbit aorta, thymol caused relaxation of norepinephrine- and potassium-induced contractions. Moreover, atropine, propranolol, indomethacin and glibenclamide did not alter the vasorelaxation caused by thymol. The results suggest that thymol is a calcium channel blocker which may explain the hypotensive and bradycardiac effects observed.
Other pharmacological effects of Trachyspermum ammi extracts include inhibition of the platelet aggregation induced by arachidonic acid, epinephrine and collagen by an ether extract of the fruits.
Aqueous and 90% ethanolic extracts were studied for their anti-reproductive potential in rats, orally dosed for 10 days after insemination, and were found to have moderate teratologic potential. The aqueous extract of the fruits was tested for anti-oxidant properties in human erythrocyte membranes against lipid peroxidation induced by FeSO4-ascorbate, and was found to exhibit 75% inhibition. It was also found to inhibit the formation of diene, triene and tetraene conjugates in human erythrocyte membrane. In addition, the essential oil also showed excellent anti-oxidant effects of stored sunflower and soya bean oil.
An aqueous extract of the fruits was evaluated for the effects on gastric acid secretion in anaesthetized rats. The stomach of anaesthetized rats was perfused at 0.15 ml/min with the extract or with acetylcholine (1 µg/ml or 10 µg/ml). Acute gastric mucosal injury was induced by leaving aspirin, at 125 mg/kg, in the stomach for 2 h before perfusion. The extract increased acid secretion, while atropine abolished the acid secretion induced by acetylcholine and significantly reduced acid induction by the extract.
Adulterations and Substitutes
Thymol was formerly important as an essential oil, but is now mainly produced synthetically. Natural thymol can also be obtained from Thymus vulgaris L. or the thymol type of Ocimum gratissimum L.
Description
An annual, erect, aromatic herb, 25-B60(B-140) cm tall, stems striate, glabrous, usually strongly branched. Leaves alternate, pinnately compound; petiole long, sheathing; blade in outline ovate-elliptical, up to 13 cm x 12 cm, 2—3-pinnate, segments linear to narrowly oblong, up to 2 mm long. Inflorescence a terminal or axillary, compound umbel, up to 6 cm in diameter; peduncle 1B-10 cm long; involucral bracts 3B-6, linear-lanceolate, sometimes divided; rays 5—9(—17) per umbel, 0.5-B3 cm long, up to 2 cm in fruit; pedicels (secondary rays) 8—15(-B25), 1—6 mm long, bracteoles 4B-7; flowers actinomorphic, 5-merous, bisexual, calyx teeth 0.5 mm long, fleshy, petals obcordate, 0.6—0.7 mm long, apex inflexed, white; stamens 5, radiating, anthers reddish-brown, ovary inferior, densely white hairy, styles 2, stigma globose. Fruit a flattened, subglobose schizocarp, splitting into 2 hairy, 1-seeded mericarps, 2 mm x 1 mm, each mericarp with 5 longitudinal ribs, on ribs with broad, warty trichomes, each mericarp with 4—6 oil ducts. Seed tiny, ovoid, embryo straight, endosperm copious, grey. Seedling with epigeal germination; cotyledons oblanceolate, 5—15 mm x 1—2 mm, base attenuate, slightly sheathing, first leaf simple, blade ovate in outline, deeply divided into 3 lobes, each repeatedly incised.
Image
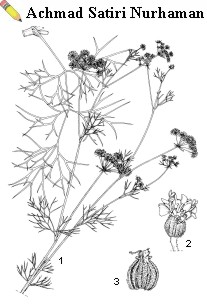 | Trachyspermum ammi (L.) Sprague ex Turrill - 1, flowering stem and basal leaf; 2, flower, one petal removed; 3, fruit |
Growth and Development
In India, flowering of Trachyspermum ammi starts 2—4 months after sowing, and fruits are ripe 2 months later. It is cross-pollinated for 70—80%, and pollination is primarily done by bees. As the plants are widely branched, flowering occurs unevenly, thus fruits mature unevenly, which makes them difficult to harvest.
Other Botanical Information
Trachyspermum consists of 15—20 species, distributed from tropical and North-East Africa to Central Asia, India and western China. Several species are cultivated in South-East Asia. Trachyspermum is closely related to Carum, but the genera have been separated based on differences in life cycle and pubescence of fruits. A general revision of Trachyspermum and related genera is needed, as the taxonomy of the group is very confusing. In India, several cultivars of Trachyspermum ammi are known, which are mainly distinguishable by the size of the fruit.
Ecology
In South-East Asia, Trachyspermum ammi is grown on the hills, up to 750 m altitude. In Ethiopia, it is cultivated at 1700—2200 m altitude, but when grown at 2000 m fruit setting is less satisfactory. The climate of Central Europe is not considered suitable for Trachyspermum ammi, and cultivation ceased centuries ago, although it is still found here and there in the wild. Trachyspermum ammi prefers not too heavy, loamy soils, but can be grown on all types of soils, although wet-rice-growing soils are considered unsuitable as they promote vegetative development.
Propagation and planting
Trachyspermum ammi is propagated by seed. Good seed is difficult to obtain, as many fruits for sale on the market are empty. In India, germination takes 10—15 days, while in Ethiopia it may take as long as 1 month. Cool and cloudy weather and gentle rain after sowing are important for the establishment of the crop. In India, sowing is from September to November, depending on the regions, at the rate of 2.3—3.5 kg/ha. Trachyspermum ammi is often broadcast, as a sole crop or intercropped with grain crops or other Umbelliferae. The light fruits need to be covered by a thin layer of soil for good germination, and to prevent flushing. For sowing, the fruits are sometimes mixed with rice husks to ensure even distribution. In India, the fruits are sown in rows 45 cm apart and 30 cm between plants.
An efficient and reliable protocol for in vitro regeneration of Trachyspermum ammi was established, and complete plantlet formation was achieved using shoot tips as explants on Murashige & Skoog (MS) medium supplemented with either indole acetic acid (IAA) at 3 mg/l or naphthalene acetic acid (NAA) at 3 mg/l, within 3—4 weeks.
An efficient and reliable protocol for in vitro regeneration of Trachyspermum ammi was established, and complete plantlet formation was achieved using shoot tips as explants on Murashige & Skoog (MS) medium supplemented with either indole acetic acid (IAA) at 3 mg/l or naphthalene acetic acid (NAA) at 3 mg/l, within 3—4 weeks.
In Vitro Production of Active Compounds
Callus cultures were established from mature fruits on MS media to study thymol production in vitro. Undifferentiated white callus was obtained on MS containing 2,4-D at 3 µg/ml, and chlorophyllous callus on MS containing IAA. Addition of NAA and kinetin to the IAA-containing medium resulted in the initiation of organogenesis and the formation of semi-differentiated callus. The undifferentiated and semi-differentiated calluses contained about 0.10% and 0.17% total phenols on a dry weight basis, respectively. The thymol content of both calluses increased with time but was highest in semi-differentiated callus. Addition of monoterpenes to the medium as precursors for thymol is undesirable as they have the opposite effect.
Husbandry
Weeding of Trachyspermum ammi is important, and plants are thinned or transplanted if necessary at the first weeding. The crop is irrigated in gardens or small fields if rainfall is insufficient. Although the crop responds well to manure, it is not often fertilized.
Diseases and Pests
Trachyspermum ammi is not often affected by diseases and pests in the field. Several leaf spot diseases may occur, caused by Alternaria dauci and Cercospora sp., and also root rot, caused by Sclerotium rolfsii. In India, the larvae of the chalcid fly Systole albipennis feed on the fruits and can cause about 10% yield loss. When stored, the fruits are often attacked by the drug-store beetle Stegobium paniceum and by rot-causing fungi like Aspergillus flavus, Aspergillus niger and Penicillium nigricans. It is also susceptible to root-knot nematodes.
Harvesting
The fruits of Trachyspermum ammi are often harvested before they are fully ripe, to prevent loss due to shattering. The essential oil quality of these fruits is considered the same as that of the ripe fruits.
Yield
In India, yields of traditionally grown Trachyspermum ammi are higher under dry rain-fed conditions than under irrigation, and amount to about 225 kg/ha of fruits. Improved cultivars may give yields of 1.2—2.2 t/ha and in 1997 an improved cultivar yielded 82 kg/ha essential oil.
Handling After Harvest
The fruits of Trachyspermum ammi are used fresh or dried and stored for later use. The fruits can be ground before storage.
Genetic Resources and Breeding
Trachyspermum ammi is not commonly cultivated in South-East Asia, and could therefore be in danger of genetic erosion. No germplasm collections are known to exist, although populations in India show a large genotypic diversity.
Breeding experiments have been carried out in India to obtain types with higher yield of fruits and oil, resulting in cultivars with an oil content twice as high.
Breeding experiments have been carried out in India to obtain types with higher yield of fruits and oil, resulting in cultivars with an oil content twice as high.
Prospects
The potential for Trachyspermum ammi cultivation in the cooler regions of South-East Asian countries needs further investigation. On account of its low toxicity, further research on the properties of the oil as a hypotensive agent is recommended.
Literature
Boskabady, M.H. & Shaikhi, J., 2000. Inhibitory effect of Carum copticum on histamine (H1) receptors of isolated guinea-pig tracheal chains. Journal of Ethnopharmacology 69(3): 217—227.
Buwalda, P., 1949. Umbelliferae. In: van Steenis, C.G.G.J. (Editor): Flora Malesiana. Series 1, Vol. 4. Noordhoff-Kolff, Djakarta, Indonesia. pp. 113-B140.
Jansen, P.C.M., 1981. Spices, condiments and medicinal plants in Ethiopia, their taxonomy and agricultural significance. Pudoc, Wageningen, the Netherlands. pp. 111—120.
Sharma, M., Batra, A., Sardana, J., Ali, D.J. & Ajita C.S., 1997. An efficient and reliable protocol for single step in vitro regeneration of Trachyspermum ammi. Journal of Phytological Research 10(1/2): 93—96.
Siemonsma, J.S. & Jansen, P.C.M., 1999. Trachyspermum roxburghianum (DC.) H. Wolff. In: de Guzman, C.C. & Siemonsma, J.S. (Editors): Plant Resources of South-East Asia No 13. Spices. Backhuys Publishers, Leiden, the Netherlands. pp. 223—225.
Valsaraj, R., Pushpangadan, P., Smitt, U.W., Adsersen, A. & Nyman, U., 1997. Antimicrobial screening of selected medicinal plants from India. Journal of Ethnopharmacology 58(2): 75—83.
Buwalda, P., 1949. Umbelliferae. In: van Steenis, C.G.G.J. (Editor): Flora Malesiana. Series 1, Vol. 4. Noordhoff-Kolff, Djakarta, Indonesia. pp. 113-B140.
Jansen, P.C.M., 1981. Spices, condiments and medicinal plants in Ethiopia, their taxonomy and agricultural significance. Pudoc, Wageningen, the Netherlands. pp. 111—120.
Sharma, M., Batra, A., Sardana, J., Ali, D.J. & Ajita C.S., 1997. An efficient and reliable protocol for single step in vitro regeneration of Trachyspermum ammi. Journal of Phytological Research 10(1/2): 93—96.
Siemonsma, J.S. & Jansen, P.C.M., 1999. Trachyspermum roxburghianum (DC.) H. Wolff. In: de Guzman, C.C. & Siemonsma, J.S. (Editors): Plant Resources of South-East Asia No 13. Spices. Backhuys Publishers, Leiden, the Netherlands. pp. 223—225.
Valsaraj, R., Pushpangadan, P., Smitt, U.W., Adsersen, A. & Nyman, U., 1997. Antimicrobial screening of selected medicinal plants from India. Journal of Ethnopharmacology 58(2): 75—83.
Other Selected Sources
[16] Aftab, K., Atta ur Rahman, Usmanghani, K., 1995. Blood pressure lowering action of active principle from Trachyspermum ammi (L.) Sprague. Phytomedicine 2(1): 35—40.
[19] Agrawal, R. & Patwardhan, M.V., 1987. Thymol - a secondary product from callus cultures of ajowan (Carum copticum). Journal of Food Science and Technology (India) 24(6): 322—324.
[135] Burkill, I.H., 1966. A dictionary of the economic products of the Malay Peninsula. Revised reprint. 2 volumes. Ministry of Agriculture and Co-operatives, Kuala Lumpur, Malaysia. Vol. 1 (A—H) pp. 1—1240, Vol. 2 (I—Z) pp. 1241—2444.
[215] Council of Scientific and Industrial Research, 1948—1976. The wealth of India: a dictionary of Indian raw materials & industrial products. 11 volumes. Publications and Information Directorate, New Delhi, India.
[234] Dave, Y.S. & Menon, A.R.S., 1989. Systematic and comparative value of fruit architecture in Trachyspermum species. Feddes Repertorium 100(5—6): 251—256.
[407] Heyne, K., 1950. De nuttige planten van Indonesië [The useful plants of Indonesia]. 3rd Edition. 2 volumes. W. van Hoeve, 's-Gravenhage, the Netherlands/Bandung, Indonesia. 1660 + CCXLI pp.
[462] Ijaz, A., Raie, M.Y. & Akhtar, M.W., 1994. Lipid studies of Carum copticum seed oil. Pakistan Journal of Scientific and Industrial Research 37(4): 150—152.
[726] Nath, D., Sethi, N., Singh, R.K. & Jain, A.K., 1992. Commonly used Indian abortifacient plants with special reference to their teratologic effects in rats. Journal of Ethnopharmacology 36(2): 147—154.
[767] Oyen, L.P.A. & Nguyen Xuan Dung (Editors), 1999. Plant Resources of South-East Asia No 19. Essential-oil plants. Backhuys Publishers, Leiden, the Netherlands. 277 pp.
[810] Quisumbing, E., 1978. Medicinal plants of the Philippines. Katha Publishing Co., Quezon City, the Philippines. 1262 pp.
[936] Singh, S., Singh, V.K. & Singh, D.K., 1998. Molluscicidal activity of some common spice plants. Biological Agriculture & Horticulture 14(3): 237—249.
[961] Srivastava, K.C., 1988. Extract of a spice — omum (Trachyspermum ammi) — shows antiaggregatory effects and alters arachidonic acid metabolism in human platelets. Prostaglandins, Leukotrienes and Essential Fatty Acids 33(1): 1—6.
[969] Sujatha, R. & Srinivas, L., 1995. Modulation of lipid peroxidation by dietary components. Toxicology In Vitro 9(3): 231—236.
[991] Tare, V. & Sharma, R.N., 1991. Larvicidal activity of some tree oils and their common chemical constituents against mosquitoes. Pesticide Research Journal 3(2): 169—172.
[1034] Vasudevan, K., Vembar, S., Veeraraghavan, K. & Haranath, P.S., 2000. Influence of intragastric perfusion of aqueous spice extracts on acid secretion in anesthetized albino rats. Indian Journal of Gastroenterology 19(2): 53—56.
[19] Agrawal, R. & Patwardhan, M.V., 1987. Thymol - a secondary product from callus cultures of ajowan (Carum copticum). Journal of Food Science and Technology (India) 24(6): 322—324.
[135] Burkill, I.H., 1966. A dictionary of the economic products of the Malay Peninsula. Revised reprint. 2 volumes. Ministry of Agriculture and Co-operatives, Kuala Lumpur, Malaysia. Vol. 1 (A—H) pp. 1—1240, Vol. 2 (I—Z) pp. 1241—2444.
[215] Council of Scientific and Industrial Research, 1948—1976. The wealth of India: a dictionary of Indian raw materials & industrial products. 11 volumes. Publications and Information Directorate, New Delhi, India.
[234] Dave, Y.S. & Menon, A.R.S., 1989. Systematic and comparative value of fruit architecture in Trachyspermum species. Feddes Repertorium 100(5—6): 251—256.
[407] Heyne, K., 1950. De nuttige planten van Indonesië [The useful plants of Indonesia]. 3rd Edition. 2 volumes. W. van Hoeve, 's-Gravenhage, the Netherlands/Bandung, Indonesia. 1660 + CCXLI pp.
[462] Ijaz, A., Raie, M.Y. & Akhtar, M.W., 1994. Lipid studies of Carum copticum seed oil. Pakistan Journal of Scientific and Industrial Research 37(4): 150—152.
[726] Nath, D., Sethi, N., Singh, R.K. & Jain, A.K., 1992. Commonly used Indian abortifacient plants with special reference to their teratologic effects in rats. Journal of Ethnopharmacology 36(2): 147—154.
[767] Oyen, L.P.A. & Nguyen Xuan Dung (Editors), 1999. Plant Resources of South-East Asia No 19. Essential-oil plants. Backhuys Publishers, Leiden, the Netherlands. 277 pp.
[810] Quisumbing, E., 1978. Medicinal plants of the Philippines. Katha Publishing Co., Quezon City, the Philippines. 1262 pp.
[936] Singh, S., Singh, V.K. & Singh, D.K., 1998. Molluscicidal activity of some common spice plants. Biological Agriculture & Horticulture 14(3): 237—249.
[961] Srivastava, K.C., 1988. Extract of a spice — omum (Trachyspermum ammi) — shows antiaggregatory effects and alters arachidonic acid metabolism in human platelets. Prostaglandins, Leukotrienes and Essential Fatty Acids 33(1): 1—6.
[969] Sujatha, R. & Srinivas, L., 1995. Modulation of lipid peroxidation by dietary components. Toxicology In Vitro 9(3): 231—236.
[991] Tare, V. & Sharma, R.N., 1991. Larvicidal activity of some tree oils and their common chemical constituents against mosquitoes. Pesticide Research Journal 3(2): 169—172.
[1034] Vasudevan, K., Vembar, S., Veeraraghavan, K. & Haranath, P.S., 2000. Influence of intragastric perfusion of aqueous spice extracts on acid secretion in anesthetized albino rats. Indian Journal of Gastroenterology 19(2): 53—56.
Author(s)
G.H. Schmelzer
Correct Citation of this Article
Schmelzer, G.H., 2001. Trachyspermum ammi (L.) Sprague ex Turrill. In: van Valkenburg, J.L.C.H. and Bunyapraphatsara, N. (Editors): Plant Resources of South-East Asia No 12(2): Medicinal and poisonous plants 2. PROSEA Foundation, Bogor, Indonesia. Database record: prota4u.org/prosea

All texts are licensed under a Creative Commons Attribution-Noncommercial-Share Alike 3.0 Netherlands License
This license does not include the illustrations (Maps,drawings,pictures); these remain all under copyright.