Record Number
2982
PROSEA Handbook Number
11: Auxiliary plants
Taxon
Acacia auriculiformis A. Cunn. ex Benth.
Protologue
London Journ. Bot. 1: 377 (1842).
Family
LEGUMINOSAE - MIMOSOIDEAE
Chromosome Numbers
2n = 26
Synonyms
Racosperma auriculiforme (A. Cunn. ex Benth.) Pedley (1986). Acacia auriculaeformis A. Cunn. ex Benth. is a formerly commonly used orthographic variant of Acacia auriculiformis.
Vernacular Names
Northern black wattle (Australian standard trade name), ear-pod wattle, tan wattle (En). Earleaf acacia (Am). Indonesia: akasia, ki hia (Sundanese). Malaysia: akasia kuning. Papua New Guinea: Papua wattle. Philippines: Japanese acacia, auri. Cambodia: smach'té:hs. Thailand: krathin-narong (Bangkok).
Origin and Geographic Distribution
Natural stands of Acacia auriculiformis are found in Australia (Cape York Peninsula, Queensland, northern areas of the Northern Territory), south-western Papua New Guinea and Indonesia (Irian Jaya, Kai Islands). The domestication of Acacia auriculiformis began about 50 years ago. It is planted widely in tropical Asia, with extensive plantings in China and India. In western Malesia it has also become naturalized. It is planted to a lesser extent in Africa and South America.
Uses
Acacia auriculiformis is a major source of firewood, its dense wood and high energy value contributing to its popularity. It provides very good charcoal which glows well with little smoke and does not spark. In agroforestry systems, Acacia auriculiformis appears to be used mainly for fuelwood.
Its superficial and densely matted root system makes Acacia auriculiformis suitable for stabilizing eroded land. It has been used widely in revegetation and rehabilitation of degraded land in Indonesia. It is planted to provide shelter along beaches and sea fronts. Because of its tolerance of poor soils it is used in reforestation of tin and bauxite mine tailings. Its phyllodes provide a very good, long-lasting mulch.
The wood of Acacia auriculiformis is extensively used for paper pulp and small saw timber. It makes attractive furniture. Crooked and multiple stems have long restricted its use as poles or other forms of timber that require to be of reasonable length, but genotypes with good trunk form have been identified and are now widely planted. Plantation-grown trees have been found to be very promising for the production of unbleached kraft pulp and high quality neutral sulphite semi-chemical pulp. Large-scale plantations have already been established e.g. in Kerala, India, for the production of pulp. The bark is collected locally for use as tanning material.
The foliage is not a good fodder and is rarely, if ever, browsed by cattle. Lac insects have been found on trees of Acacia auriculiformis in India, but it is probably not a good substitute for traditional host species. An edible mushroom, Tylopylus fellus, is common in plantations of Acacia auriculiformis in Thailand. The flowers are a source of pollen for honey producing bees.
The dense dark green foliage which remains throughout the dry season makes it an excellent shade tree. It is a popular ornamental tree with attractive, bright yellow flowers. The flowers are marketed in Burma (Myanmar) as altar flowers.
Its superficial and densely matted root system makes Acacia auriculiformis suitable for stabilizing eroded land. It has been used widely in revegetation and rehabilitation of degraded land in Indonesia. It is planted to provide shelter along beaches and sea fronts. Because of its tolerance of poor soils it is used in reforestation of tin and bauxite mine tailings. Its phyllodes provide a very good, long-lasting mulch.
The wood of Acacia auriculiformis is extensively used for paper pulp and small saw timber. It makes attractive furniture. Crooked and multiple stems have long restricted its use as poles or other forms of timber that require to be of reasonable length, but genotypes with good trunk form have been identified and are now widely planted. Plantation-grown trees have been found to be very promising for the production of unbleached kraft pulp and high quality neutral sulphite semi-chemical pulp. Large-scale plantations have already been established e.g. in Kerala, India, for the production of pulp. The bark is collected locally for use as tanning material.
The foliage is not a good fodder and is rarely, if ever, browsed by cattle. Lac insects have been found on trees of Acacia auriculiformis in India, but it is probably not a good substitute for traditional host species. An edible mushroom, Tylopylus fellus, is common in plantations of Acacia auriculiformis in Thailand. The flowers are a source of pollen for honey producing bees.
The dense dark green foliage which remains throughout the dry season makes it an excellent shade tree. It is a popular ornamental tree with attractive, bright yellow flowers. The flowers are marketed in Burma (Myanmar) as altar flowers.
Properties
Phyllodes decompose slowly in comparison with those of other leguminous trees like Paraserianthes falcataria (L.) Nielsen or Leucaena leucocephala (Lamk) de Wit; this has been attributed to a lower N content and a harder cuticula. In West Java, phyllodes used as a mulch were 95% decomposed after 16 months.
The bark contains sufficient tannin (13—25%) for commercial exploitation and contains 6—14% of a natural dye suitable for the soga-batik industry.
Leaves have an in vitro dry matter digestibility of 33—37%. Per 100 g dry matter, leaves contain: crude protein 13—16 g, P 0.06—0.11 g, K 0.45—0.72 g, Ca 0.52—0.77 g, Mg 0.18—0.24 g.
The wood of Acacia auriculiformis contains 66% cellulose, 31% lignin, 16% pentosans and 1.5% ash. Flavonoid substances are present. It is heavy with more than 70% of the volume being heartwood. The sapwood is yellow; the heartwood light brown to dark red, straight grained and reasonably durable. It has a fine to medium texture and is often attractively figured. The density is 490—840 kg/m3 at 15% moisture content. The fibre is relatively short, about 0.85 mm long and 0.2 ?m in diameter. The physical and mechanical properties of the wood as compared to teak as standard are considered high. The wood is easy to work, takes a good polish and finishes well. Boards, however, have a tendency to split when sawn. The energy value of the wood is 20 000—20 500 kJ/kg. Tests on Acacia auriculiformis charcoal carried out in Thailand, gave an energy value of 32 300 kJ/kg and a density of 404 kg/m3. It gave little or no smoke or sparks. The weight of 1000 seeds is 15—30 g.
The bark contains sufficient tannin (13—25%) for commercial exploitation and contains 6—14% of a natural dye suitable for the soga-batik industry.
Leaves have an in vitro dry matter digestibility of 33—37%. Per 100 g dry matter, leaves contain: crude protein 13—16 g, P 0.06—0.11 g, K 0.45—0.72 g, Ca 0.52—0.77 g, Mg 0.18—0.24 g.
The wood of Acacia auriculiformis contains 66% cellulose, 31% lignin, 16% pentosans and 1.5% ash. Flavonoid substances are present. It is heavy with more than 70% of the volume being heartwood. The sapwood is yellow; the heartwood light brown to dark red, straight grained and reasonably durable. It has a fine to medium texture and is often attractively figured. The density is 490—840 kg/m3 at 15% moisture content. The fibre is relatively short, about 0.85 mm long and 0.2 ?m in diameter. The physical and mechanical properties of the wood as compared to teak as standard are considered high. The wood is easy to work, takes a good polish and finishes well. Boards, however, have a tendency to split when sawn. The energy value of the wood is 20 000—20 500 kJ/kg. Tests on Acacia auriculiformis charcoal carried out in Thailand, gave an energy value of 32 300 kJ/kg and a density of 404 kg/m3. It gave little or no smoke or sparks. The weight of 1000 seeds is 15—30 g.
Description
Tree up to 30 m tall with trunk up to 12 m long and 50 cm in diameter, often smaller with crooked stem and heavily branched; branchlets angular, glabrous. Bark grey or brown, more or less smooth in young trees, becoming rough and fissured with age. Phyllodes curved or falcate, 10—16 cm x 1—3 cm, glabrous, greyish-green, 3—4 longitudinal veins prominent, usually not yellowish, running towards the lower margin or in the middle near the base, with many, fine, crowded, somewhat anastomosing secondary veins; pulvinus 4—6 mm, with at the top a distinct, swollen gland with small rimmed orifice. Inflorescence an axillary, somewhat interrupted spike, 7—10 cm long, growing in pairs; peduncle 5—8 mm long; flowers 5-merous, bisexual, tiny, sessile, golden-yellow, fragrant; calyx tubular, 0.7—1 mm long, shortly lobed, glabrous; corolla 1.7—2 mm long, about 2 times as long as the calyx; stamens many, about 3 mm long; ovary densely pubescent. Fruit a flat pod, about 6.5 cm x 1—2.5 cm, cartilaginous or woody, brown, glaucous, transversely veined with undulate margins, initially straight, on maturity becoming very twisted with irregular spirals. Seed broadly ovate to elliptical, 4—6 mm x 3—4 mm, shiny black, hard, transversely attached, encircled by a long red or orange funicle; areole large, almost closed.
Image
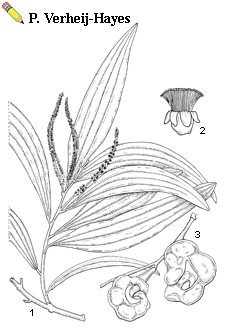 | Acacia auriculiformis A. Cunn. ex Benth. - 1, flowering branch; 2, flower; 3, pods |
Growth and Development
Under favourable conditions, seedlings grow quickly and reach a height of 25—30 cm in 3—4 months, 6 m in 2 years, and 6—12 m in 3 years. Young seedlings produce 2—3 bipinnate leaves, soon followed by phyllodes. Phyllodes are retained during the dry season; their average life is about 1 year in West Java.
Under favourable conditions, Acacia auriculiformis grows into a tree, 25—30 m tall, with a straight stem dominant for a greater part of the tree height. Where it is introduced, e.g. in India, it is commonly a low tree, 8—12 m tall, much branched and with a crooked stem. Inbreeding in introduced populations with a narrow genetic base has been suggested as a major cause of poor form.
Flowering usually starts within 2 years after sowing. Though flowering occurs throughout the year, there is usually a distinct peak flowering season. These periods vary considerably with locality. In Java, for example, peak flowering is from March to June. Seeds mature in 4—5 months and store well. Germination rate is adequate after storage in airtight containers at room temperature for 18 months or for several years in air-conditioned rooms.
Nodulation and atmospheric nitrogen fixation by Acacia auriculiformis are profuse under good growing conditions, but this potential can only be reached where soil fertility, especially in terms of P content, is adequate. In trials in the Philippines, 52—66% of nitrogen uptake has been derived from nitrogen fixation. Nodulation is profuse, with a range of Rhizobium and Bradyrhizobium species. Ecto-mycorrhizal fungi (Thelephora spp.) and endo-mycorrhizal fungi (Glomus etunicatum, Glomus macrocarpum and Gigaspora margarita) have been shown to form effective associations.
Under favourable conditions, Acacia auriculiformis grows into a tree, 25—30 m tall, with a straight stem dominant for a greater part of the tree height. Where it is introduced, e.g. in India, it is commonly a low tree, 8—12 m tall, much branched and with a crooked stem. Inbreeding in introduced populations with a narrow genetic base has been suggested as a major cause of poor form.
Flowering usually starts within 2 years after sowing. Though flowering occurs throughout the year, there is usually a distinct peak flowering season. These periods vary considerably with locality. In Java, for example, peak flowering is from March to June. Seeds mature in 4—5 months and store well. Germination rate is adequate after storage in airtight containers at room temperature for 18 months or for several years in air-conditioned rooms.
Nodulation and atmospheric nitrogen fixation by Acacia auriculiformis are profuse under good growing conditions, but this potential can only be reached where soil fertility, especially in terms of P content, is adequate. In trials in the Philippines, 52—66% of nitrogen uptake has been derived from nitrogen fixation. Nodulation is profuse, with a range of Rhizobium and Bradyrhizobium species. Ecto-mycorrhizal fungi (Thelephora spp.) and endo-mycorrhizal fungi (Glomus etunicatum, Glomus macrocarpum and Gigaspora margarita) have been shown to form effective associations.
Other Botanical Information
Acacia auriculiformis is closely related to Acacia aulacocarpa A. Cunn. ex Benth. and Acacia leptocarpa A. Cunn. ex Benth. It is difficult to distinguish from Acacia aulacocarpa, which has phyllodes with non-anastomosing veins, while those of Acacia auriculiformis are somewhat anastomosing. Acacia leptocarpa has long, non-contorted pods with seeds disposed longitudinally, and the veins in the phyllodes are spaced more widely.
The natural habitat of Acacia auriculiformis overlaps with that of several closely related species. Natural hybrids with Acacia mangium Willd. and Acacia leptocarpa occur. The hybrids with Acacia mangium are intermediate between the two parents in flower, fruit, and seed characteristics and in physical and mechanical wood properties. They inherit the better stem straightness of Acacia mangium and the self-pruning ability and better stem roundness of Acacia auriculiformis. Their growth is sometimes more vigorous and resistance to heart rot is better.
The natural habitat of Acacia auriculiformis overlaps with that of several closely related species. Natural hybrids with Acacia mangium Willd. and Acacia leptocarpa occur. The hybrids with Acacia mangium are intermediate between the two parents in flower, fruit, and seed characteristics and in physical and mechanical wood properties. They inherit the better stem straightness of Acacia mangium and the self-pruning ability and better stem roundness of Acacia auriculiformis. Their growth is sometimes more vigorous and resistance to heart rot is better.
Ecology
Acacia auriculiformis occurs in the humid to sub-humid lowland tropics, growing naturally in narrow strips along river banks but also on coastal dunes, tidal flats, saline lagoons and floodplains. Individual trees may be widely scattered in savanna woodland or swamp forest dominated by tall Melaleuca spp. It occurs naturally from sea level to 400 m. In plantations in Nepal and Zimbabwe it has done well up to 1000 m altitude. In its natural range, the mean maximum temperature of the hottest month is 32—38°C and the mean minimum temperature of the coldest month 12—20°C. Rainfall varies between 760 mm in the Northern Territory (Australia) and 2000 mm in Papua New Guinea; its distribution is monsoonal and the dry season may last 6 months. Plantations have been established in areas with as little as 650 mm to over 6000 mm rainfall annually. Frost does not occur in its natural range, but elsewhere light frost is tolerated. It does not tolerate shade. Wind tolerance is low, as branches break easily in strong winds.
Acacia auriculiformis is exceptionally tolerant of adverse soil conditions. In Papua New Guinea it grows well on well-drained acid soils and on imperfectly drained heavy clay soils subject to temporary or prolonged waterlogging and flooding. Soils in its natural range in Australia include dune sands, black cracking clays, and alluvium derived from sandstone or laterite. The pH usually ranges from 4.5—6.5, but in the Northern Territory it grows on beach sands with a pH of 8—9, as well as on the spoils of uranium mines with pH 3. It is highly tolerant of soil salinity. In an experiment in Thailand, it continued growing under saline conditions ranging from 0.15 to 7.25 dS/m, in both wet and dry soils.
Acacia auriculiformis is exceptionally tolerant of adverse soil conditions. In Papua New Guinea it grows well on well-drained acid soils and on imperfectly drained heavy clay soils subject to temporary or prolonged waterlogging and flooding. Soils in its natural range in Australia include dune sands, black cracking clays, and alluvium derived from sandstone or laterite. The pH usually ranges from 4.5—6.5, but in the Northern Territory it grows on beach sands with a pH of 8—9, as well as on the spoils of uranium mines with pH 3. It is highly tolerant of soil salinity. In an experiment in Thailand, it continued growing under saline conditions ranging from 0.15 to 7.25 dS/m, in both wet and dry soils.
Propagation and planting
The use of local, often inbred seed sources should be discouraged to avoid inbreeding depression and the resulting poor tree form. Seeds picked at physiological maturity do not show dormancy, but mature seeds require a pregermination treatment, such as immersion in boiling water for 1—2 minutes followed by soaking in cold water overnight or soaking in warm water for 24 hours. After suitable treatment, germination starts about 6 days after sowing and typically exceeds 75%.
Seeds are mostly sown in nurseries. Direct sowing is also possible. Sowing from the air has sometimes been successful, but site preparation prior to sowing is required. Seedlings in the nursery require little attention and there are no serious diseases and pests. Newly emerged seedlings should receive 50% shade; once established, 70% full sunlight is optimal. In general, 3—4 months are needed to raise transplantable seedlings that are 25 cm tall. Inoculation with rhizobia and mycorrhizae is rarely necessary, unless seedlings are raised in sterilized media or planted in highly degraded soils or mine spoils.
Methods of vegetative propagation through juvenile cuttings have been developed and are now a routine and simple operation. Trees can be pollarded to produce cuttings.
The optimum planting density is not clearly established. Most current plantings employ spacings of 2—4 m x 2—4 m, the closer spacing being more suitable for firewood and pulp plantations. In China, spacings of 1—1.5 m x 1.5—2 m are favoured by farmers producing fuelwood and poles.
Seeds are mostly sown in nurseries. Direct sowing is also possible. Sowing from the air has sometimes been successful, but site preparation prior to sowing is required. Seedlings in the nursery require little attention and there are no serious diseases and pests. Newly emerged seedlings should receive 50% shade; once established, 70% full sunlight is optimal. In general, 3—4 months are needed to raise transplantable seedlings that are 25 cm tall. Inoculation with rhizobia and mycorrhizae is rarely necessary, unless seedlings are raised in sterilized media or planted in highly degraded soils or mine spoils.
Methods of vegetative propagation through juvenile cuttings have been developed and are now a routine and simple operation. Trees can be pollarded to produce cuttings.
The optimum planting density is not clearly established. Most current plantings employ spacings of 2—4 m x 2—4 m, the closer spacing being more suitable for firewood and pulp plantations. In China, spacings of 1—1.5 m x 1.5—2 m are favoured by farmers producing fuelwood and poles.
Husbandry
Removal of lower branches of young plants has been suggested as a means of improving stem form and of reducing the incidence of multiple stems. Acacia auriculiformis responds well to pollarding. Tree age, stump diameter and height are important factors in sprouting, although their effects are not well understood and warrant detailed investigation. Plantings in Imperata grasslands have survived fires, but are generally too severely damaged to make Acacia auriculiformis a suitable tree for Imperata control.
The effect of intercropping with annual crops varies. Increased tree growth has been found with kenaf (Hibiscus cannabinus L.), upland rice and groundnut in Thailand, reduced growth with maize in Cameroon.
The effect of intercropping with annual crops varies. Increased tree growth has been found with kenaf (Hibiscus cannabinus L.), upland rice and groundnut in Thailand, reduced growth with maize in Cameroon.
Diseases and Pests
No serious diseases and pests occur. Seedlings in the nursery are reported to be infested by powdery mildew and rust, but these do not usually cause serious damage. In nurseries and young plantations in Indonesia growth rates have been impaired by the rust Uromyces digitatus. Root rot caused by Ganoderma lucidum is reported from India. A beetle (Sinoxylon sp.) can girdle young stems and branches, causing them to break. This insect is of concern, because the tree will develop multiple leaders if the main stem is damaged, and the length of the bole will be reduced. Experimental results suggest that Acacia auriculiformis has some resistance to termites.
Harvesting
Acacia auriculiformis does coppice well, but it does not sprout vigorously or prolifically. Better results are obtained when the stump is cut at a height of 0.6—1 m above the ground. Tree age, stump diameter and season of cutting also influence coppicing ability. It responds well to pollarding.
Yield
Under favourable conditions, trees may reach a height of 15 m in 5 years, and produce an average annual wood increment of 15—20 m3/ha over 10—12 years, the age at which it is usually harvested. On very poor or severely eroded soils, mean annual increment drops to 8—12 m3/ha. Under rainfall conditions of 1000—1400 mm/year and a pronounced dry season in West Bengal, India, the mean annual increment was only 2—6 m3/ha. Differences between provenances are large. On a well-drained site in Thailand receiving about 1500 mm rainfall annually, a provenance from Balamuk (Papua New Guinea) produced a total above-ground biomass of 135 t/ha in 3 years, while a provenance from Springvale (Australia) reached only 60 t/ha. On a regosol overlaying marl in West Java in a region with a rainfall of 2700 mm/year, the biomass increment from year 3 to 4 was 15.7 t reaching 96.1 m3 in year 4; stemwood made up 60% of the total above-ground biomass; stem diameter increased from 12.2 cm to 13.6 cm; litter production was 10.7 t/year of which 6.4 t were leaves.
Up to 500 g of seed per tree has been collected.
Up to 500 g of seed per tree has been collected.
Genetic Resources
The Australian Tree Seed Centre of the Commonwealth Scientific and Industrial Research Organization (CSIRO) in Canberra maintains seed stocks of representative provenances from throughout the natural range of Acacia auriculiformis. Comprehensive living collections are currently maintained on Melville Island, Australia, and in provenance trials in China, Thailand and elsewhere.
Breeding
High levels of genetic variation exist in Acacia auriculiformis. Intra and inter-population genetic variation is considerable and important in initial selections in domestication programmes. Generally, 3 distinct groups can be distinguished, corresponding to their geographic distribution in Queensland, Northern Territory, and Papua New Guinea, respectively. International provenance trials were established in 1989 to examine the extent of genotype x environment interactions. Results from Australia and Thailand show that provenances from Queensland have a higher proportion of straight stems. Several countries have genetic improvement programmes which aim to improve this characteristic. Collection of seeds from phenotypically superior trees, field progeny trials, and seedling-seed orchards have produced promising results.
Natural hybridization of Acacia auriculiformis with Acacia leptocarpa and Acacia mangium has been observed in both natural stands and plantations. Many hybrids show desirable characteristics, such as vigour, fine branching and tendency for strong apical dominance, which will eventually lead to a tree with single stem and a long, straight, branchless bole.
Natural hybridization of Acacia auriculiformis with Acacia leptocarpa and Acacia mangium has been observed in both natural stands and plantations. Many hybrids show desirable characteristics, such as vigour, fine branching and tendency for strong apical dominance, which will eventually lead to a tree with single stem and a long, straight, branchless bole.
Prospects
Few species can match the ability of Acacia auriculiformis to grow on harsh sites in the tropics. Although it grows slower than some other species under optimal conditions, its fast growth rate, the ability to fix atmospheric nitrogen and its tolerance of infertile, acid, alkaline, saline and seasonally waterlogged soils, and of moderately dry seasons make it a most useful tree for the rehabilitation of degraded lands. Its ease of cultivation and multiple uses make it suitable for growing by farmers. Straight-stemmed forms have outstanding prospects for industrial plantations to produce paper pulp and other timber products. The use of Acacia auriculiformis as a parent of hybrids is of great potential, perhaps even exceeding the potential of the species itself.
Literature
Awang, K. & Taylor, D.A. (Editors), 1992. Tropical acacias in East Africa and the Pacific. Proceedings of a first meeting of the Consultative Group for Research and Development of Acacias (COGREDA) held in Phuket, Thailand, 1-3 June 1992. Winrock International Institute for Agricultural Research, Bangkok, Thailand. 106 pp.
Carron, L.T. & Aken, K.M. (Editors), 1992. Breeding technologies for tropical acacias. Proceedings of a workshop held in Tawau, Sabah, Malaysia, 1-4 July 1991. ACIAR Proceedings No 37. Australian Centre for International Agricultural Research, Canberra, Australia. 132 pp.
Logan, A.F., 1987. Australian acacias for pulpwood. In: Turnbull, J.W. (Editor): Australian acacias for developing countries. Proceedings of an international workshop held in Gympie, Queensland, Australia, 4-7 August 1986. ACIAR Proceedings No 16. Australian Centre for International Agricultural Research, Canberra, Australia. pp. 89-94.
Nielsen, I.C., 1992. Mimosaceae (Leguminosae - Mimosoideae). Acacia. In: de Wilde, W.J.J.O., Nooteboom, H.P. & Kalkman, C. (Editors): Flora Malesiana. Series 1, Vol. 11. Foundation Flora Malesiana, Leiden, the Netherlands. pp. 34-64.
Pedley, L., 1978. A revision of Acacia Mill. in Queensland. Austrobaileya 1(2): 172-173.
Pinyopusarerk, K., 1990. Acacia auriculiformis: an annotated bibliography. Winrock International Institute for Agricultural Research, Bangkok, Thailand and Australian Centre for International Agricultural Research, Canberra, Australia. 154 pp.
Rufelds, C.W., 1987. Quantitative comparison of Acacia mangium Willd. versus hybrid A. auriculiformis. Forest Research Centre Publication No 40, Sabah, Malaysia. 22 pp.
Turnbull, J.W. (Editor), 1991. Advances in tropical acacia research. Proceedings of an international workshop held in Bangkok, Thailand, 11-15 February 1991. ACIAR Proceedings No 35. Australian Centre for International Agricultural Research, Canberra, Australia. 234 pp.
Vercoe, T.K., 1989. Fodder value of selected Australian trees and shrubs. In: Boland, D.J. (Editor): Trees for the tropics - growing Australian multipurpose trees and shrubs in developing countries. ACIAR Monograph No 10. Australian Centre for International Agricultural Research, Canberra, Australia. pp. 187-192.
Wiersum, K.F. & Ramlan, A., 1982. Cultivation of Acacia auriculiformis on Java, Indonesia. Commonwealth Forestry Review 61: 135-144.
Carron, L.T. & Aken, K.M. (Editors), 1992. Breeding technologies for tropical acacias. Proceedings of a workshop held in Tawau, Sabah, Malaysia, 1-4 July 1991. ACIAR Proceedings No 37. Australian Centre for International Agricultural Research, Canberra, Australia. 132 pp.
Logan, A.F., 1987. Australian acacias for pulpwood. In: Turnbull, J.W. (Editor): Australian acacias for developing countries. Proceedings of an international workshop held in Gympie, Queensland, Australia, 4-7 August 1986. ACIAR Proceedings No 16. Australian Centre for International Agricultural Research, Canberra, Australia. pp. 89-94.
Nielsen, I.C., 1992. Mimosaceae (Leguminosae - Mimosoideae). Acacia. In: de Wilde, W.J.J.O., Nooteboom, H.P. & Kalkman, C. (Editors): Flora Malesiana. Series 1, Vol. 11. Foundation Flora Malesiana, Leiden, the Netherlands. pp. 34-64.
Pedley, L., 1978. A revision of Acacia Mill. in Queensland. Austrobaileya 1(2): 172-173.
Pinyopusarerk, K., 1990. Acacia auriculiformis: an annotated bibliography. Winrock International Institute for Agricultural Research, Bangkok, Thailand and Australian Centre for International Agricultural Research, Canberra, Australia. 154 pp.
Rufelds, C.W., 1987. Quantitative comparison of Acacia mangium Willd. versus hybrid A. auriculiformis. Forest Research Centre Publication No 40, Sabah, Malaysia. 22 pp.
Turnbull, J.W. (Editor), 1991. Advances in tropical acacia research. Proceedings of an international workshop held in Bangkok, Thailand, 11-15 February 1991. ACIAR Proceedings No 35. Australian Centre for International Agricultural Research, Canberra, Australia. 234 pp.
Vercoe, T.K., 1989. Fodder value of selected Australian trees and shrubs. In: Boland, D.J. (Editor): Trees for the tropics - growing Australian multipurpose trees and shrubs in developing countries. ACIAR Monograph No 10. Australian Centre for International Agricultural Research, Canberra, Australia. pp. 187-192.
Wiersum, K.F. & Ramlan, A., 1982. Cultivation of Acacia auriculiformis on Java, Indonesia. Commonwealth Forestry Review 61: 135-144.
Author(s)
J.W. Turnbull & Kamis Awang
Correct Citation of this Article
Turnbull, J.W. & Awang, K., 1997. Acacia auriculiformis A. Cunn. ex Benth.. In: Faridah Hanum, I & van der Maesen, L.J.G. (Editors): Plant Resources of South-East Asia No 11: Auxiliary plants. PROSEA Foundation, Bogor, Indonesia. Database record: prota4u.org/prosea

All texts are licensed under a Creative Commons Attribution-Noncommercial-Share Alike 3.0 Netherlands License
This license does not include the illustrations (Maps,drawings,pictures); these remain all under copyright.