Record Number
617
PROSEA Handbook Number
16: Stimulants
Taxon
Theobroma cacao L.
Protologue
Sp. pl.: 782 (1753).
Family
STERCULIACEAE
Chromosome Numbers
2n = 20
Vernacular Names
Cocoa, cacao (En). Cacaoyer (plant), cacao (product) (Fr). Indonesia: cokelat, kakao. Malaysia: koko. Papua New Guinea: diwai kakau (Kilimeri). Philippines: cacao. Burma (Myanmar): kokoe. Thailand: koko. Vietnam: ca cao.
Note: In the current English literature the terms 'cacao' for the plant and 'cocoa' for its products have generally been replaced by 'cocoa' to encompass both meanings.
Note: In the current English literature the terms 'cacao' for the plant and 'cocoa' for its products have generally been replaced by 'cocoa' to encompass both meanings.
Origin and Geographic Distribution
The primary centres of genetic diversity of Theobroma cacao are in the upper basin of the Amazon and its headwaters (in Peru, Ecuador, Colombia and Brazil). Natural cocoa populations are also present in the understorey of the tropical rain forests in the lower Amazon basin, as well as along the Orinoco river in Venezuela and in the Guyanas. These populations in the upper and lower Amazon basin form the Forastero group and are distinct from the 'Criollo' cocoa, which according to archaeological evidence must have been widely cultivated since ancient times by the Maya and other peoples in tropical Central America. However, the geographically isolated Criollo populations are presumed to have an Amazon-basin origin like all other cocoa forms. In Central America the valuable cocoa beans were used as currency in the local trade and also to prepare an invigorating beverage, called 'cacahuatl', by boiling a mix of ground roasted cocoa beans with maize, vanilla and chili peppers. They also played a role in rituals in Maya and neighbouring civilizations. Soon after conquering Mexico in the first years of the 16th Century, the Spanish discovered that a beverage based on ground roasted cocoa beans and sugar was more pleasing to their palate and this 'cocoa' or 'chocolate' drink was soon to become popular with the wealthy upper classes of Europe. The disintegration of the Central American civilizations caused a rapid decline in the traditional supply of cocoa beans. Cocoa cultivation was then introduced into the Caribbean area, first in Venezuela and on Trinidad around 1525 and subsequently to several other islands. Criollo dominated cocoa production until the end of the 18th Century, when Forastero cocoa began to be cultivated on a large scale in the Brazilian state of Bahia and in Ecuador. The Amelonado Forastero cocoa in Bahia originated in the lower Amazon basin, whereas 'Nacional' Forastero cocoa of Ecuador originated in the upper Amazon basin. Trinitario, a natural hybrid between Criollo and Venezuelan Forastero, replaced the earlier Criollo cocoa on Trinidad after 1800. The vast areas of cocoa production in West Africa (Ghana, Nigeria, Cameroon and Ivory Coast) are based on Amelonado (from Bahia) and Trinitario introduced during the 19th Century and Forastero from the upper Amazon basin introduced shortly after 1945. The Criollo cocoa of Sulawesi and Java can be traced back to the few seedlings taken from Central America to the Philippines by the Spanish around the year 1600. Other cocoa introductions of importance to the 20th Century cocoa industries of Asia, in particular in Indonesia, Malaysia and Papua New Guinea, included Trinitario and Forastero material from various Caribbean and South American origins. World cocoa production increased from 18 000 t in 1850 (all from tropical America) to 370 000 t in 1920 (tropical America 50%, West Africa 48% and Asia 2%) and 2 500 000 t in 1990 (tropical America 27%, West Africa 56% and Asia 17%).
Uses
Until the middle of the 19th Century about the only cocoa product consumed in Europe and elsewhere was a chocolate drink. The cocoa press invented by C.J. van Houten in the Netherlands around 1828 to extract most of the cocoa butter, and the process to manufacture milk chocolate invented by M.D. Peter in Switzerland in 1876, had major impacts on the diversification of cocoa products and consequent increase in world consumption. The preferred cocoa powders for drinking chocolate contain 20—25% fat and are derived from cocoa liquor usually treated with alkali before pressing to improve flavour and dispersability (the 'Dutching' process, also invented by van Houten). Low fat (10—13%) powders are applied in confectionery, biscuits, ice-creams and other chocolate-flavoured products. Chocolate is made from a mix of cocoa liquor and butter to which sugar (and sometimes milk) has been added. Most cocoa butter is used to manufacture chocolates, but a minor volume also finds applications in cosmetic and pharmaceutical products. Milk chocolate in a variety of forms (bars, fillings, coatings) is still the backbone of the world cocoa industry.
Production and International Trade
Average annual production of cocoa worldwide over the period 1995—1998 was about 2.6 million t from a total of 6.1 million ha in 40 countries in tropical America (20% of world production), West Africa (63%) and Asia (17%). The following six countries produced 80% of all the cocoa: Ivory Coast 39%, Ghana 13%, Indonesia 11%, Brazil 7%, Nigeria 6% and Malaysia 4%.
In Indonesia production increased hundredfold from 3000 t in 1976 to an estimated 332 000 t in 1997 (610 000 ha), mainly due to cocoa expansion in Sulawesi and Sumatra. In Malaysia production rose from 10 000 t in 1974 to 240 000 t in 1990, but fell again to about 100 000 t (200 000 ha) in 1997, because of large increases in labour costs and falling world market prices. In Papua New Guinea production has remained stable at about 30 000 t per year (90 000 ha). The Philippines produces some 5000 t cocoa annually from 9000 ha.
About 85% of the world production is exported, mostly to Europe (the Netherlands being the largest importer, followed by Germany, the United Kingdom and France, among others) and the United States, as cured beans but also after conversion into cocoa liquor and butter.
Cocoa processing industries in 15 producing countries account for about 30% of world cocoa grindings. The present cocoa grinding capacity in Malaysia is 100 000 t per year, in Indonesia 70 000 t, in Singapore 60 000 t (all imports) and in the Philippines 15 000 t (exceeding local production).
In West Africa cocoa is a smallholders' crop with only 5% grown on plantations, mainly in Ivory Coast and Cameroon. Large-scale plantations are predominant in many tropical American countries, e.g. 55% in Brazil, but also in Asia, e.g. 80% in Malaysia and 40% of the area in Indonesia.
The trade in cocoa beans in most producing countries operates under a free marketing system except in Ghana (control by a marketing board). Almost all internationally traded cocoa is subject to conditions of cocoa merchants associations established in major consumer countries. The value of the world trade in cured cocoa beans, at an average annual price of US$ 1400 per t in 1997, is estimated at US$ 3.1 billion. Cocoa prices have fluctuated between US$ 3500 per t in 1976 to an all time low of US$ 800 per t in 1992, recovered to US$ 1600 per t by the end of 1997 and once more came down to US$ 850 in 1999, in response to large variations in supply (production and stocks) against only gradually increasing demand.
Cocoa consumption appears to be much more related to consumer income than to world market prices. Per capita annual consumption (cured beans equivalent) averages 2.5 kg in Western Europe (Switzerland 4.8, Belgium 4.2, Germany 3.1 kg), 2.2 kg in the United States, 2.0 kg in Australia and 1.1 kg in Poland. However, average cocoa consumption in Japan is only 0.9 kg per inhabitant. Cocoa consumption in most producing countries is very low, except in Brazil (0.7 kg) and Mexico (0.5 kg).
The cocoa market distinguishes two main types:
— 'fine' or flavour Criollo, Trinitario and the Nacional (Ecuador) cocoa, which account for less than 5% of the total production, and
— 'bulk' or ordinary cocoa produced worldwide from Forastero cultivars.
There is usually a significant price differential between fine and bulk cocoa. On the other hand, the carefully cured cocoa from Ghana (Amelonado and hybrid Forastero) has always been regarded to be superior in flavour to cocoa from Ivory Coast, Malaysia or Indonesia. Indonesia produces about 12 000 t fine cocoa annually in Java and Papua New Guinea another 10 000 t, both from Trinitario type cocoa. The fine cocoa or 'edel kakao' from Java is also well-known as Java 'A' light-breaking cocoa due to the almost white cotyledons.
In Indonesia production increased hundredfold from 3000 t in 1976 to an estimated 332 000 t in 1997 (610 000 ha), mainly due to cocoa expansion in Sulawesi and Sumatra. In Malaysia production rose from 10 000 t in 1974 to 240 000 t in 1990, but fell again to about 100 000 t (200 000 ha) in 1997, because of large increases in labour costs and falling world market prices. In Papua New Guinea production has remained stable at about 30 000 t per year (90 000 ha). The Philippines produces some 5000 t cocoa annually from 9000 ha.
About 85% of the world production is exported, mostly to Europe (the Netherlands being the largest importer, followed by Germany, the United Kingdom and France, among others) and the United States, as cured beans but also after conversion into cocoa liquor and butter.
Cocoa processing industries in 15 producing countries account for about 30% of world cocoa grindings. The present cocoa grinding capacity in Malaysia is 100 000 t per year, in Indonesia 70 000 t, in Singapore 60 000 t (all imports) and in the Philippines 15 000 t (exceeding local production).
In West Africa cocoa is a smallholders' crop with only 5% grown on plantations, mainly in Ivory Coast and Cameroon. Large-scale plantations are predominant in many tropical American countries, e.g. 55% in Brazil, but also in Asia, e.g. 80% in Malaysia and 40% of the area in Indonesia.
The trade in cocoa beans in most producing countries operates under a free marketing system except in Ghana (control by a marketing board). Almost all internationally traded cocoa is subject to conditions of cocoa merchants associations established in major consumer countries. The value of the world trade in cured cocoa beans, at an average annual price of US$ 1400 per t in 1997, is estimated at US$ 3.1 billion. Cocoa prices have fluctuated between US$ 3500 per t in 1976 to an all time low of US$ 800 per t in 1992, recovered to US$ 1600 per t by the end of 1997 and once more came down to US$ 850 in 1999, in response to large variations in supply (production and stocks) against only gradually increasing demand.
Cocoa consumption appears to be much more related to consumer income than to world market prices. Per capita annual consumption (cured beans equivalent) averages 2.5 kg in Western Europe (Switzerland 4.8, Belgium 4.2, Germany 3.1 kg), 2.2 kg in the United States, 2.0 kg in Australia and 1.1 kg in Poland. However, average cocoa consumption in Japan is only 0.9 kg per inhabitant. Cocoa consumption in most producing countries is very low, except in Brazil (0.7 kg) and Mexico (0.5 kg).
The cocoa market distinguishes two main types:
— 'fine' or flavour Criollo, Trinitario and the Nacional (Ecuador) cocoa, which account for less than 5% of the total production, and
— 'bulk' or ordinary cocoa produced worldwide from Forastero cultivars.
There is usually a significant price differential between fine and bulk cocoa. On the other hand, the carefully cured cocoa from Ghana (Amelonado and hybrid Forastero) has always been regarded to be superior in flavour to cocoa from Ivory Coast, Malaysia or Indonesia. Indonesia produces about 12 000 t fine cocoa annually in Java and Papua New Guinea another 10 000 t, both from Trinitario type cocoa. The fine cocoa or 'edel kakao' from Java is also well-known as Java 'A' light-breaking cocoa due to the almost white cotyledons.
Properties
Forastero cocoa beans with the testa removed have the following average composition per 100 g fresh weight: water 35 g, protein 8.4 g, fat 31.3 g, starch 4.5 g, sugars 6 g, fibre 3.2 g, theobromine 2.4 g, caffeine 0.8 g, polyphenols (catechins, tannins, leuco-cyanidins and cyanidin glycosides) 5.2 g, acids (acetic and lactic) 0.6 g, inorganic salts 2.6 g. The chocolate flavour precursors are formed during fermentation, when the protein and polyphenol compounds react with hydrolytic enzymes. The astringent catechins and other polyphenols are oxidized and become insoluble. The bitter purines are partly lost by exudation during the fermentation process.
Cocoa quality factors including single bean weight (0.6—2.1 g), shell content (11—16%), cotyledon colour (white-purple), butter content (50—65% of dry weight) and butter hardness (harder is more valuable) can be physically measured. However, the most important factors determining cocoa quality are flavour and mouthfeel of the final products, developed during fermenting and after roasting, and these require reliable sensory evaluation. More than 60 aromatic compounds have been found to contribute to cocoa flavour.
Cocoa powder mixed with milk and sugar makes a very nutritious drink and the presence of theobromine and caffeine gives a mildly stimulating action. Plain and milk chocolates have a high calorific value (>2000 kJ/100 g) and provide a concentrated food with excellent keeping quality. A typical bar of milk chocolate may contain 15% cocoa liquor, 20% cocoa butter, 22% milk solids, 40% sugar, small amounts of lecithin emulsifier and vanillin.
The 1000-seed weight (at 30% moisture content) is about 2 kg.
Cocoa quality factors including single bean weight (0.6—2.1 g), shell content (11—16%), cotyledon colour (white-purple), butter content (50—65% of dry weight) and butter hardness (harder is more valuable) can be physically measured. However, the most important factors determining cocoa quality are flavour and mouthfeel of the final products, developed during fermenting and after roasting, and these require reliable sensory evaluation. More than 60 aromatic compounds have been found to contribute to cocoa flavour.
Cocoa powder mixed with milk and sugar makes a very nutritious drink and the presence of theobromine and caffeine gives a mildly stimulating action. Plain and milk chocolates have a high calorific value (>2000 kJ/100 g) and provide a concentrated food with excellent keeping quality. A typical bar of milk chocolate may contain 15% cocoa liquor, 20% cocoa butter, 22% milk solids, 40% sugar, small amounts of lecithin emulsifier and vanillin.
The 1000-seed weight (at 30% moisture content) is about 2 kg.
Description
Evergreen tree, 4—20 m tall, in cultivation usually 4—6 m. Taproot up to 2 m long with a dense mat of lateral feeder roots up to 6 m long in upper 20 cm of the soil; roots possibly with mycorrhizal associations. Stem growth sympodial, with orthotropic subterminal shoots (chupons) and lateral branching with successive whorls (fan or 'jorquette') of (3—)5(—6) plagiotropic branches. Leaves thin-coriaceous, petiolate, arranged spirally on orthotropic, alternate on plagiotropic branches; petiole 1—10 cm long, characteristically thickened at both ends; blade subobovate-oblong to elliptical-oblong, 15—50 cm x 4—15 cm, rounded at base, subundulate along the margin, apex acuminate, pubescent on the veins; with a prominent main vein and 9—12 pairs of lateral veins. Inflorescences on the trunk and older branches (cauliflorous), usually borne on small tubercles (flower-cushions) in many-flowered fascicles; flower 5-merous, 1—1.5 cm in diameter, regular, bisexual; pedicel 0.5—1.5 cm long; sepals 5, oblong to lanceolate, 5—8 mm x 1.5—2 mm, white to reddish, reflexed; petals 5, smaller than sepals, with obovate base, expanding into concave cup-shaped pouch, upper part spatulate, pale yellow, reflexed; androecium with 5 outer, erect, pointed, ciliate staminodes and 5 inner stamens with reflexed filaments, anthers concealed in pouches of corresponding petals; pistil with 5 very short styles, connate at base. Fruit a berry-like drupe, commonly called pod, very variable in shape, from globose to cylindrical and pointed, 10—32 cm x 6—12 cm, smooth to warty, usually with 5 or 10 furrows, green, yellow, red or purplish, with fruit wall (husk) varying in thickness (up to 2 cm) and in degree of lignification, 20—60-seeded. Seeds (beans) arranged in 5 rows, with central placentation, very variable, globose to ellipsoid, 2—4 cm x 1—2 cm, embedded in mucilageous, whitish, sugary and acid pulp, developed from the outer integument of the ovule, with two convoluted cotyledons and small embryo enclosed in thin membrane (remains of endosperm), with leathery testa. Seedling with epigeal germination.
Image
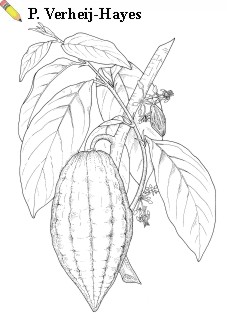 | Theobroma cacao L. - branch with flowers and fruits. |
Growth and Development
Cocoa seed is recalcitrant and there are no effective methods of prolonging seed viability in storage beyond 4—5 weeks without the seed starting to germinate. Seeds in unopened pods may remain viable for 3—4 weeks after harvesting and an inhibitor in the mucilage prevents early germination. Fresh seed placed flat about 1 cm deep in nursery pots will germinate almost immediately, with the closed cotyledons emerging above the ground within a week. A few weeks later the cotyledons open, exposing the plumule, and the first growth phase ends with the hardening-off of the first four leaves that stand out horizontally at the same level. Subsequently, leaves appear at about 6-week intervals, well spaced in a spiral arrangement. Seedlings are ready for planting in the field 4—6 months after sowing, when 40—50 cm tall. Orthotropic growth of the single stem continues until the seedling is 1—1.5 m tall, usually one year after field planting, when the first jorquette is formed. This is the product of five axial subterminal buds that grow out sideways simultaneously, whilst the apical bud ceases to function. The internodes between the side shoots are reduced so much that they grow out at the same level. These plagiotropic shoots are called 'fan' branches. Some years later an orthotropic shoot or 'chupon' may grow out from below the jorquette joint and, after growing a certain length, form another tier of fan branches. This process may be repeated several times. The dimorphic branching in Theobroma cacao is not absolute, as is the case in Coffea spp., and orthotropic shoots can be induced on mature plagiotropic stems.
First flowering starts 1.5—5 years after field planting, depending on
cultivar and ecological conditions. Cocoa is allogamous, with a unique system of gameto-sporophytic self- and cross-incompatibility controlled by a single gene with several dominant and co-dominant alleles. This self-incompatibility gene is operative in all Forastero cocoa germplasm collected from the main centres of genetic diversity (upper Amazon basin), but not always in other types, such as the self-compatible Criollo, Trinitario and Amelonado cocoa. However, even self-incompatible genotypes can be induced to self-fertilize when the pollen mix also contains pollen (called mentor pollen) from a cross-compatible genotype. This phenomenon has important consequences for seed production from biclonal seed gardens. Pollination is effected by insects, the most important being very small midges of the genus Forcipomyia, which results in 25—50% cross pollination. Pollination efficiency is low, usually less than 10%, but that is compensated for by the large number of flowers produced. Only one in every 500 flowers may develop into a mature pod, or even fewer in the presence of cross-incompatibility. Initial growth of the young fruits, called 'cherelles', is slow. Cherelle wilt, in which fruits stop growing, blacken and shrivel but remain attached to the stem, is considered to be a physiological mechanism to regulate the crop load on the tree in accordance with available assimilates. It occurs mostly during the first 2—3 months. Full pod size is attained 4—5 months after successful fertilization and another month is required for ripening.
First flowering starts 1.5—5 years after field planting, depending on
cultivar and ecological conditions. Cocoa is allogamous, with a unique system of gameto-sporophytic self- and cross-incompatibility controlled by a single gene with several dominant and co-dominant alleles. This self-incompatibility gene is operative in all Forastero cocoa germplasm collected from the main centres of genetic diversity (upper Amazon basin), but not always in other types, such as the self-compatible Criollo, Trinitario and Amelonado cocoa. However, even self-incompatible genotypes can be induced to self-fertilize when the pollen mix also contains pollen (called mentor pollen) from a cross-compatible genotype. This phenomenon has important consequences for seed production from biclonal seed gardens. Pollination is effected by insects, the most important being very small midges of the genus Forcipomyia, which results in 25—50% cross pollination. Pollination efficiency is low, usually less than 10%, but that is compensated for by the large number of flowers produced. Only one in every 500 flowers may develop into a mature pod, or even fewer in the presence of cross-incompatibility. Initial growth of the young fruits, called 'cherelles', is slow. Cherelle wilt, in which fruits stop growing, blacken and shrivel but remain attached to the stem, is considered to be a physiological mechanism to regulate the crop load on the tree in accordance with available assimilates. It occurs mostly during the first 2—3 months. Full pod size is attained 4—5 months after successful fertilization and another month is required for ripening.
Other Botanical Information
Most natural and cultivated cocoa populations show great variability, due to the allogamous nature of cocoa and a history of frequent inter-population crossing. This complicates attempts of systematic classification, but four main groups of populations within Theobroma cacao are generally distinguished:
— Criollo: rather weak-growing tree with relatively low yields, very susceptible to diseases and pests; pods longish and pointed, usually deeply furrowed and warty, thin and soft husk without lignification; green or red immature pods, ripening to yellow or red; 20—40 beans per pod; beans large and almost round in cross-section, requiring 2—3 days fermentation only; white to pale purple cotyledons.
— Forastero of lower Amazon basin (LA): mostly the Amelonado populations; large trees coming into bearing late, but high yielding; immature pods light green ripening to yellow, spherical shape with rounded or very bluntly pointed ends, generally smooth surface with shallow ridges, husk thick and hard with some lignification; beans flat and dark purple, about 40 per pod.
— Forastero of upper Amazon basin (UA): highly variable wild populations from the centres of genetic diversity; sources of plant vigour, productivity and resistance to diseases and pests; pod size and shape variable, but often rather small, pointed, sometimes with a narrow neck, often deeply furrowed and warty surface, but sometimes also smooth; husk comparatively thick and hard, often with distinct layer of sclerenchyma; immature pods always green, ripening to yellow; 30—60 beans per pod; beans small and flat; cotyledons dark purple, but occasionally light red or white.
— Trinitario: natural hybrid between Criollo and Amelonado Forastero; very heterogeneous population but generally much more vigorous and hardy than Criollo; pods variable in shape and husk thickness, smooth to warty surface; immature pods whitish, green, red or purple, ripening to yellow, orange or red; beans plump to flat; cotyledons white to dark purple.
Botanically, Theobroma cacao has been subdivided into subsp. cacao (with 4 formas) and subsp. sphaerocarpum (Chevalier) Cuatr. The former covers the Criollo group, the latter the Forastero and Trinitario groups. For cultivated plants, however, a classification into cultivar groups and cultivars would be more appropriate, but no such classification yet exists. The great cultivars of the older cocoa-growing areas, i.e. the Criollo of Central America and the Caribbean, the Forastero-Amelonados of Brazil and West Africa, the 'Nacional' of Ecuador and Trinitarios of Cameroon and Papua New Guinea, are gradually being replaced by hybrid populations obtained by crossing accessions of UA Forasteros with local selections. These hybrids accounted for 40% of the world cocoa area in the late 1990s, including 5% clonal plantings. The expansion of cocoa in Malaysia (Sabah) during the 1980s and more recently in Indonesia (Sulawesi and Sumatra)is almost entirely based on such hybrids, propagated as seedling populations or selected clones.
Theobroma cacao is the only one of the 22 species within the genus Theobroma to be cultivated worldwide for its beans. Theobroma grandiflorum (Willd. ex Sprengel) K. Schum. or 'copuaçu' is grown on a small scale in Brazil for the sweetly flavoured mucilage, which is extracted and used to prepare a refreshing sherbet. Fresh cocoa pulp is also used for similar purposes by industrial fruit processors in the state of Bahia. In Indonesia the fresh pulp is used to produce 'nata de cacao'.
— Criollo: rather weak-growing tree with relatively low yields, very susceptible to diseases and pests; pods longish and pointed, usually deeply furrowed and warty, thin and soft husk without lignification; green or red immature pods, ripening to yellow or red; 20—40 beans per pod; beans large and almost round in cross-section, requiring 2—3 days fermentation only; white to pale purple cotyledons.
— Forastero of lower Amazon basin (LA): mostly the Amelonado populations; large trees coming into bearing late, but high yielding; immature pods light green ripening to yellow, spherical shape with rounded or very bluntly pointed ends, generally smooth surface with shallow ridges, husk thick and hard with some lignification; beans flat and dark purple, about 40 per pod.
— Forastero of upper Amazon basin (UA): highly variable wild populations from the centres of genetic diversity; sources of plant vigour, productivity and resistance to diseases and pests; pod size and shape variable, but often rather small, pointed, sometimes with a narrow neck, often deeply furrowed and warty surface, but sometimes also smooth; husk comparatively thick and hard, often with distinct layer of sclerenchyma; immature pods always green, ripening to yellow; 30—60 beans per pod; beans small and flat; cotyledons dark purple, but occasionally light red or white.
— Trinitario: natural hybrid between Criollo and Amelonado Forastero; very heterogeneous population but generally much more vigorous and hardy than Criollo; pods variable in shape and husk thickness, smooth to warty surface; immature pods whitish, green, red or purple, ripening to yellow, orange or red; beans plump to flat; cotyledons white to dark purple.
Botanically, Theobroma cacao has been subdivided into subsp. cacao (with 4 formas) and subsp. sphaerocarpum (Chevalier) Cuatr. The former covers the Criollo group, the latter the Forastero and Trinitario groups. For cultivated plants, however, a classification into cultivar groups and cultivars would be more appropriate, but no such classification yet exists. The great cultivars of the older cocoa-growing areas, i.e. the Criollo of Central America and the Caribbean, the Forastero-Amelonados of Brazil and West Africa, the 'Nacional' of Ecuador and Trinitarios of Cameroon and Papua New Guinea, are gradually being replaced by hybrid populations obtained by crossing accessions of UA Forasteros with local selections. These hybrids accounted for 40% of the world cocoa area in the late 1990s, including 5% clonal plantings. The expansion of cocoa in Malaysia (Sabah) during the 1980s and more recently in Indonesia (Sulawesi and Sumatra)is almost entirely based on such hybrids, propagated as seedling populations or selected clones.
Theobroma cacao is the only one of the 22 species within the genus Theobroma to be cultivated worldwide for its beans. Theobroma grandiflorum (Willd. ex Sprengel) K. Schum. or 'copuaçu' is grown on a small scale in Brazil for the sweetly flavoured mucilage, which is extracted and used to prepare a refreshing sherbet. Fresh cocoa pulp is also used for similar purposes by industrial fruit processors in the state of Bahia. In Indonesia the fresh pulp is used to produce 'nata de cacao'.
Ecology
Cocoa is a typical crop of the tropical lowlands which can be grown at higher altitudes if other conditions are favourable.
Annual rainfall of 1500—2500 mm, with no more than three consecutive months with less than 100 mm, and temperatures between 30—32°C mean maximum and 18—21°C mean minimum are optimal (absolute minimum not below 10°C). Light is an important factor affecting the growth and development of cocoa. Wild cocoa grows as an understorey of tropical forests and cocoa is often cultivated under shade. The leaves have a very low light saturation point and a low photosynthetic rate, which declines when leaves are exposed to above-optimal light levels. Large areas of South-East Asia have conditions favourable for optimal cocoa production. In areas without a dry season, cocoa has been shown to develop more quickly than in the major production areas of West Africa where during certain months of the year growth is arrested by drought. Climatic conditions should, however, be considered in relation to soil properties. Soils with a large capacity to store moisture can compensate for periodic lack of rain, while excessive rainfall will cause fewer problems on well-drained soils.
Cocoa requires deep, well-drained, fertile soils and is more demanding than rubber or oil palm. Criteria for appropriate cocoa soils are: at least 1.5 m deep, clay content 30—40%, a topsoil with at least 2% organic carbon, a cation exchange capacity of 120 mmol/kg and a base saturation of 35%.
Soils meeting these requirements are in the orders of well-drained Entisols (alluvial soils), deep and well-drained Inceptisols (volcanic and other origins), red or yellowish Ultisols and Alfisols (mineral-rich soils under forest). In Malaysia (Sabah) and Papua New Guinea (New Britain, Bougainville Island) cocoa thrives on chemically rich Inceptisols of volcanic origin. In Peninsular Malaysia and North Sumatra most cocoa is grown on Ultisols; however, cocoa was successfully established on poorly-drained Inceptisols after water management of the soils was improved. In Sulawesi cocoa is expanding on Entisols and Ultisols.
Annual rainfall of 1500—2500 mm, with no more than three consecutive months with less than 100 mm, and temperatures between 30—32°C mean maximum and 18—21°C mean minimum are optimal (absolute minimum not below 10°C). Light is an important factor affecting the growth and development of cocoa. Wild cocoa grows as an understorey of tropical forests and cocoa is often cultivated under shade. The leaves have a very low light saturation point and a low photosynthetic rate, which declines when leaves are exposed to above-optimal light levels. Large areas of South-East Asia have conditions favourable for optimal cocoa production. In areas without a dry season, cocoa has been shown to develop more quickly than in the major production areas of West Africa where during certain months of the year growth is arrested by drought. Climatic conditions should, however, be considered in relation to soil properties. Soils with a large capacity to store moisture can compensate for periodic lack of rain, while excessive rainfall will cause fewer problems on well-drained soils.
Cocoa requires deep, well-drained, fertile soils and is more demanding than rubber or oil palm. Criteria for appropriate cocoa soils are: at least 1.5 m deep, clay content 30—40%, a topsoil with at least 2% organic carbon, a cation exchange capacity of 120 mmol/kg and a base saturation of 35%.
Soils meeting these requirements are in the orders of well-drained Entisols (alluvial soils), deep and well-drained Inceptisols (volcanic and other origins), red or yellowish Ultisols and Alfisols (mineral-rich soils under forest). In Malaysia (Sabah) and Papua New Guinea (New Britain, Bougainville Island) cocoa thrives on chemically rich Inceptisols of volcanic origin. In Peninsular Malaysia and North Sumatra most cocoa is grown on Ultisols; however, cocoa was successfully established on poorly-drained Inceptisols after water management of the soils was improved. In Sulawesi cocoa is expanding on Entisols and Ultisols.
Propagation and planting
Most cocoa is established from seed produced in bi-clonal gardens by open or controlled pollination. Hand pollination is essential to increase and direct seed production and to obtain 100% legitimate hybrid seed, even when the clones are self-incompatible. Vegetative propagation by rooted cuttings, grafting, side-grafting or budding is routinely applied everywhere to multiply selected parents for breeding programmes and elite clones for establishing seed gardens. Green-patch budding in the nursery on the hypocotyl stem of 3-week-old rootstock, and side-grafting in the field to convert mature cocoa plantings are applied in Malaysia for large-scale multiplication of commercial clonal cultivars. Traditionally, vegetative propagation was applied in Indonesia to multiply the Java fine cocoa (DR) clones, but clones of hybrid Forastero cocoa are now also being introduced, along with seed of hybrids from bi-clonal gardens. Advanced techniques of micropropagation through tissue culture or embryogenesis have so far had limited success.
Seedlings and young plants from cuttings or budding are raised in a shaded nursery, usually in polythene bags. Fields are established with 4—6-month-old nursery plants at densities of 1100—1200 trees/ha, or wider spacings when growing conditions are exceptionally favourable (e.g. on East New Britain, Papua New Guinea). In Sabah, experimental high density plantings with semi-dwarf clones have given very high early yields; however, because of high establishment and maintenance costs their commercial advantage is still questionable.
Young trees need shade to reduce irradiance, to buffer the micro-environment and to achieve the right shape and habit of the trees. Once the canopy has closed, the need for shade is reduced. Only under most favourable conditions of soil and nutrient supply can cocoa be grown without shade. It is normally necessary to retain some shade to reduce moisture stress and incidence of insect and fungus damage in order to prolong the economic life of plantations. Shade can be provided either by thinning forest or by planting shade trees. Shade trees are common in South-East Asia, the main ones being seedless Leucaena leucocephala (Lamk) de Wit (Indonesia) and Gliricidia sepium (Jacq.) Kunth ex Walp. (Malaysia and Indonesia). Often, hedges of leguminous shrubs and banana trees are used for temporary side protection between rows and as a source of mulch. Cocoa is also grown as an intercrop under coconut. The availability of large plantations of old tall coconuts has largely contributed to the rapid expansion of cocoa in South-East Asia.
Seedlings and young plants from cuttings or budding are raised in a shaded nursery, usually in polythene bags. Fields are established with 4—6-month-old nursery plants at densities of 1100—1200 trees/ha, or wider spacings when growing conditions are exceptionally favourable (e.g. on East New Britain, Papua New Guinea). In Sabah, experimental high density plantings with semi-dwarf clones have given very high early yields; however, because of high establishment and maintenance costs their commercial advantage is still questionable.
Young trees need shade to reduce irradiance, to buffer the micro-environment and to achieve the right shape and habit of the trees. Once the canopy has closed, the need for shade is reduced. Only under most favourable conditions of soil and nutrient supply can cocoa be grown without shade. It is normally necessary to retain some shade to reduce moisture stress and incidence of insect and fungus damage in order to prolong the economic life of plantations. Shade can be provided either by thinning forest or by planting shade trees. Shade trees are common in South-East Asia, the main ones being seedless Leucaena leucocephala (Lamk) de Wit (Indonesia) and Gliricidia sepium (Jacq.) Kunth ex Walp. (Malaysia and Indonesia). Often, hedges of leguminous shrubs and banana trees are used for temporary side protection between rows and as a source of mulch. Cocoa is also grown as an intercrop under coconut. The availability of large plantations of old tall coconuts has largely contributed to the rapid expansion of cocoa in South-East Asia.
Husbandry
Weeding is needed during establishment, but once the canopy has closed, lack of light will prevent weed growth. Seedling trees need no pruning during the first 2—3 years. Later, low-hanging branches should be pruned to facilitate harvesting and spraying for disease and pest control. A leaf area index of about 4 is needed to ensure optimal light interception and light penetration to the lower layers of the canopy. Vertical growth is usually restricted to the first jorquette. If the first jorquette is formed too low (below a height of 1.5 m), the tree is allowed to make a second one. To retain trees at the desired height, chupons should be removed at regular intervals.
Clonal plantings derived from plagiotropic twigs and branches require intensive pruning within one year after planting to shape the trees and reduce excessive lateral growth to maintain satisfactory yield levels. However, mature clonal trees can be induced to produce chupons allowing conversion into the seedling type of growth with one vertical stem.
Fertilizer requirements depend on yields, which are usually much higher in large estates than in smallholdings. An exception are the high fertilizer rates applied by smallholder cocoa farmers of South Sulawesi, who also achieve record yields. Nutrients removed by 1 t of cured cocoa beans are 20 kg N, 4 kg P and 10 kg K. Rates and types of fertilizer needed depend on soil fertility, age of trees, yields and shade. Lightly shaded and unshaded cocoa requires more fertilizers, especially nitrogen, than shaded cocoa. This is related to the fact that the larger leaf area, greater photosynthetic activity and higher yield of cocoa under high irradiance can only be maintained if trees are well provided with nutrients. As a general guide, per ha mature cocoa needs 50—100 kg N, 25 kg P, 75 kg K and, sometimes, 15 kg Mg per year. The highest nitrogen rate is intended for lightly shaded or unshaded cocoa. Unfortunately, leaf analysis has limited value as a diagnostic aid in cocoa nutrition when it is not combined with soil analysis and fertilizer trials. Detailed fertilizer recommendations are well documented.
Clonal plantings derived from plagiotropic twigs and branches require intensive pruning within one year after planting to shape the trees and reduce excessive lateral growth to maintain satisfactory yield levels. However, mature clonal trees can be induced to produce chupons allowing conversion into the seedling type of growth with one vertical stem.
Fertilizer requirements depend on yields, which are usually much higher in large estates than in smallholdings. An exception are the high fertilizer rates applied by smallholder cocoa farmers of South Sulawesi, who also achieve record yields. Nutrients removed by 1 t of cured cocoa beans are 20 kg N, 4 kg P and 10 kg K. Rates and types of fertilizer needed depend on soil fertility, age of trees, yields and shade. Lightly shaded and unshaded cocoa requires more fertilizers, especially nitrogen, than shaded cocoa. This is related to the fact that the larger leaf area, greater photosynthetic activity and higher yield of cocoa under high irradiance can only be maintained if trees are well provided with nutrients. As a general guide, per ha mature cocoa needs 50—100 kg N, 25 kg P, 75 kg K and, sometimes, 15 kg Mg per year. The highest nitrogen rate is intended for lightly shaded or unshaded cocoa. Unfortunately, leaf analysis has limited value as a diagnostic aid in cocoa nutrition when it is not combined with soil analysis and fertilizer trials. Detailed fertilizer recommendations are well documented.
Diseases and Pests
Every year about 40% of the world cocoa crop is lost due to diseases. Black pod caused by Phytophthora palmivora is of worldwide significance, but the more aggressive P. macrokarya is restricted to West Africa. P. palmivora is also the pathogen of bark canker, which is of particular importance in Papua New Guinea. Witches' broom disease (Crinipellis perniciosa) and moniliasis or frosty pod rot (Moniliophthora theobromae) are two fungal diseases that have co-evolved with the crop in Latin America but have not been observed outside that continent. After large areas of cocoa were established in Africa, the trees became infected by the swollen shoot virus, transmitted by mealybugs from several indigenous tree species. Swollen shoot had a devastating effect on the cocoa industry in Ghana. A fungal disease specific to South-East Asia is vascular streak dieback caused by Oncobasidium theobromae. It causes dieback of branches, especially in young trees. The disease was first reported in Papua New Guinea in 1960 but is now also present in Malaysia, the Philippines and Indonesia, where it is particularly severe in areas of high rainfall. A combination of chemical control with fungicides (e.g. triazoles) and sanitation pruning is recommended, but the existence of high levels of host resistance in certain Forastero accessions offers opportunities for a more effective way of combating this disease. In South-East Asia, especially Trinitario type cocoa is also susceptible to anthracnose (Colletotrichum gloeosporioides).
Mirids are the most important insect pest of cocoa on a world scale. All regions have their specific mirids, causing severe damage to twigs, branches and young pods. In West Africa the most important pests are mirids of the genera Distantiella and Sahlberghella. In South-East Asia, mirids of the genus Helopeltis are a major pest. Biological control with the ant-mealybug complex has been successfully re-introduced to regulate Helopeltis.
The cocoa pod-borer, the larva of a small moth (Conopomorpha cramerella), is the most serious insect pest of cocoa in South-East Asia. It bores into the cocoa pod and by feeding on the placental tissues it reduces or prevents normal bean development. During most of its life, the insect is protected within the pod and so is difficult to control. At the beginning of the 20th Century, the cocoa pod-borer largely destroyed the early cocoa industries of North Sulawesi and Java. The current decline of cocoa production in Malaysia can be partly attributed to this pod-borer. It is increasingly a problem in Indonesian (Java, Sumatra, Moluccas, Sulawesi) and Philippine (Mindanao) cocoa.
A variety of insect pests are important during crop establishment, because they destroy the apical bud and delay or prevent canopy formation. Larvae of the moth Tirocola plagiata, the cocoa army-worm, cause extensive damage to young plants, especially in Papua New Guinea. Both rats and squirrels can account for a considerable part of total crop losses in South-East Asia. The only effective control measures are baiting and trapping.
Mirids are the most important insect pest of cocoa on a world scale. All regions have their specific mirids, causing severe damage to twigs, branches and young pods. In West Africa the most important pests are mirids of the genera Distantiella and Sahlberghella. In South-East Asia, mirids of the genus Helopeltis are a major pest. Biological control with the ant-mealybug complex has been successfully re-introduced to regulate Helopeltis.
The cocoa pod-borer, the larva of a small moth (Conopomorpha cramerella), is the most serious insect pest of cocoa in South-East Asia. It bores into the cocoa pod and by feeding on the placental tissues it reduces or prevents normal bean development. During most of its life, the insect is protected within the pod and so is difficult to control. At the beginning of the 20th Century, the cocoa pod-borer largely destroyed the early cocoa industries of North Sulawesi and Java. The current decline of cocoa production in Malaysia can be partly attributed to this pod-borer. It is increasingly a problem in Indonesian (Java, Sumatra, Moluccas, Sulawesi) and Philippine (Mindanao) cocoa.
A variety of insect pests are important during crop establishment, because they destroy the apical bud and delay or prevent canopy formation. Larvae of the moth Tirocola plagiata, the cocoa army-worm, cause extensive damage to young plants, especially in Papua New Guinea. Both rats and squirrels can account for a considerable part of total crop losses in South-East Asia. The only effective control measures are baiting and trapping.
Harvesting
The length of the harvesting season depends on genotype and climatic conditions. In many cocoa areas in South-East Asia, where rainfall and its distribution are favourable, the cropping season is extended over the whole year with only 2—3 months having less than 10% of annual yield. The practice of leaving pods to rot on the trees during these months since it is not worthwhile harvesting them should be discouraged because diseases and pests of pods can multiply during this period. Ripe pods, recognizable by the colour change, are harvested at 7—10 day intervals with various types of sharp knives, care being taken not to damage the flower cushions. The time for harvest is not very critical, as the beans remain in good condition in the attached ripe pods for 2—3 weeks. However, overripe beans may germinate in the pods, and unripe beans give poor germination.
Yield
Average annual cocoa yields are about 400 kg/ha of cured beans, with national annual average yields per ha ranging from 300 kg (Ghana) and 550 kg (Ivory Coast) to 650—700 kg (Malaysia, Indonesia). Yields of mature cocoa on estates in South-East Asia are usually 1500—2000 kg/ha, but higher yields (3000—4000 kg/ha) are no exception. Cocoa smallholders on the fertile alluvial soils of South Sulawesi are reported to achieve 2000 kg/ha. The seed constitutes about 25% by fresh weight of mature pods.
Handling After Harvest
Harvested pods are opened and the beans and mucilage removed and transferred to wooden boxes for fermentation during 2—4 days for Criollo and Trinitario, or 4—6 days for Forastero cocoa. In West Africa most of the cocoa is fermented in heaps or in baskets covered with banana leaves. During fermentation the mucilage round the seeds is removed, precursors to the chocolate flavours are developed and most of the astringency disappears. The fermented beans are dried in the sun or in artificial driers to a moisture content of 6—7%. The drying process should not be too rapid in order to allow a gradual curing and further development of the flavour and the brown chocolate colour. Very slow drying may induce mouldiness and off-flavours. Bulk cocoa produced by smallholders in Indonesia (Sulawesi, Sumatra) is often unfermented and rapidly dried on concrete platforms or in artificial driers. This cocoa is of medium quality, but price discount on the world market is insufficient to force farmers to ferment the cocoa properly. In Java, beans are sometimes washed between fermentation and drying, to remove any remnants of pulp adhering to the shell. The resulting clean and attractive appearance and low proportion of shell (8—10%) are a trade mark of the fine-grade Indonesian Trinitario cocoa.
After drying, the beans are bagged and transported to overseas markets in containers. However, the importance of bulk storage and handling is increasing. Beans can safely be stored for 2—3 years. Storage in the tropics requires special precautions to prevent mould, insect damage and deterioration. Further stages of processing include roasting, shelling, liquor grinding, pressing and mixing. At present already 30% of all cocoa is produced into liquor, butter and powder before exportation to consumer countries.
After drying, the beans are bagged and transported to overseas markets in containers. However, the importance of bulk storage and handling is increasing. Beans can safely be stored for 2—3 years. Storage in the tropics requires special precautions to prevent mould, insect damage and deterioration. Further stages of processing include roasting, shelling, liquor grinding, pressing and mixing. At present already 30% of all cocoa is produced into liquor, butter and powder before exportation to consumer countries.
Genetic Resources
Cocoa germplasm collection in the centres of high genetic diversity started in earnest with the two expeditions in 1937 and 1942 to Peru by F.J. Pound of Trinidad, who was in search of host resistance to the witches' broom disease. These semi-wild UA Forastero accessions were distributed from Trinidad to all major cocoa research centres in the world after 1945 — such as the IMC (Iquitos mixed calabacillo), NA (Nanay), PA (Parinari), SCA (Scavina) and P (Pound) clones or their seedling offspring — and still form the major UA component of modern cocoa hybrids.
Some 42 expeditions have been mounted since 1949 to collect wild and other important cocoa germplasm (UA and LA Forastero and Criollo) in primary and secondary centres of genetic diversity in Brazil (19), Ecuador (9), Colombia (4), Venezuela (3), Guyana and French Guiana (2), Peru (2), Guatemala (1) and Belize (1). Large collections are maintained by the International Cocoa Genebank, Trinidad (ICG,T) with 2500 accessions, the 'Comissão Executiva do Plano da Lavoura Cacaueira' (CEPLAC) in Brazil with more than 2000 accessions at Belem, and the 'Centro Agronómico Tropical de Investigación y Enseñanza' (CATIE) in Costa Rica with 700 (Criollo and Trinitario) accessions. There are also smaller collections of original germplasm in Ecuador, French Guiana and Colombia, while most public and private cocoa breeding centres in tropical America, Africa and Asia have their own working collections of wild and improved cocoa germplasm.
The ICGT and CATIE in particular have major projects on detailed taxonomic and agronomic characterization of cocoa germplasm. This information together with data from other cocoa research centres has been assembled in the International Cocoa Germplasm Database (ICGD) coordinated by the University of Reading in the United Kingdom. The ICGD currently holds descriptive data on more than 14 000 genotypes, including 7000 unique wild accessions. The International Plant Genetic Resources Institute (IPGRI) started a global project on cocoa germplasm conservation and utilization in 1997, with financial support from public and private institutions, including the cocoa industry.
International cocoa germplasm exchanges (seed and budwood) require a 2-year period of quarantine to contain inadvertent spread of diseases and pests. Cocoa quarantine facilities are available at the University of Reading (U.K.), at the 'Centre de Coopération Internationale en Recherche Agronomique pour le Développement' (CIRAD) in Montpellier (France) and in Barbados (ICG,T).
Some 42 expeditions have been mounted since 1949 to collect wild and other important cocoa germplasm (UA and LA Forastero and Criollo) in primary and secondary centres of genetic diversity in Brazil (19), Ecuador (9), Colombia (4), Venezuela (3), Guyana and French Guiana (2), Peru (2), Guatemala (1) and Belize (1). Large collections are maintained by the International Cocoa Genebank, Trinidad (ICG,T) with 2500 accessions, the 'Comissão Executiva do Plano da Lavoura Cacaueira' (CEPLAC) in Brazil with more than 2000 accessions at Belem, and the 'Centro Agronómico Tropical de Investigación y Enseñanza' (CATIE) in Costa Rica with 700 (Criollo and Trinitario) accessions. There are also smaller collections of original germplasm in Ecuador, French Guiana and Colombia, while most public and private cocoa breeding centres in tropical America, Africa and Asia have their own working collections of wild and improved cocoa germplasm.
The ICGT and CATIE in particular have major projects on detailed taxonomic and agronomic characterization of cocoa germplasm. This information together with data from other cocoa research centres has been assembled in the International Cocoa Germplasm Database (ICGD) coordinated by the University of Reading in the United Kingdom. The ICGD currently holds descriptive data on more than 14 000 genotypes, including 7000 unique wild accessions. The International Plant Genetic Resources Institute (IPGRI) started a global project on cocoa germplasm conservation and utilization in 1997, with financial support from public and private institutions, including the cocoa industry.
International cocoa germplasm exchanges (seed and budwood) require a 2-year period of quarantine to contain inadvertent spread of diseases and pests. Cocoa quarantine facilities are available at the University of Reading (U.K.), at the 'Centre de Coopération Internationale en Recherche Agronomique pour le Développement' (CIRAD) in Montpellier (France) and in Barbados (ICG,T).
Breeding
The early 20th Century cocoa breeding programmes in Java and Trinidad achieved progress in plant vigour and yield, in combination with good bean quality, by clonal selection within progeny of Trinitario type hybrids (Djati Roenggo (DR) and Imperial College Selections (ICS) clones in Java and Trinidad respectively). The considerably higher yield potential of hybrids of Amelonado and UA Forastero or even between UA Forastero types, first confirmed in Ghana in the 1960s, eventually led to the reciprocal recurrent selection schemes starting from genetically distinct subpopulations now adopted by most cocoa breeding programmes (e.g. Ivory Coast, Brazil, Malaysia and Indonesia).
Priorities in national cocoa breeding have shifted to host resistance to the globally important black pod and regionally important diseases, such as witches' broom in Brazil, swollen shoot virus in Ghana and vascular-streak dieback in South-East Asia. Disease resistance has to be a component of a fully integrated breeding programme, as resistance is only meaningful to the cocoa grower in combination with acceptable agronomic characteristics. In the case of vascular-streak dieback, high levels of host resistance have been found in some UA Forastero accessions (e.g. SCA and NA) and progress in breeding resistant hybrid cultivars is good. Host resistance to black pod exists in UA Forastero germplasm (e.g. SCA, PA and P) but levels are usually low, making it necessary to accumulate resistance genes in recombination crosses between progenitors prior to integration in the main breeding programme. Recent development of early preselection tests by artificial inoculation of leaf disks (Trinidad, Ivory Coast) should accelerate selection. An ecologically acceptable solution to the serious menace of the pod borer to the South-East Asian cocoa industry may be found in an integrated approach including biological control (e.g. egg parasitoids of the genus Trichogrammatoidea) and breeding for partial resistance or for a preventive mechanism, such as the presence of a sclerotic layer in the husk barring penetration of the borer in the pods of certain UA Forastero accessions.
There is evidence that in cocoa the genetic variance for components of all major agronomic characters is mainly due to additive gene effects. This should result in more efficient accumulation of favourable genes within breeding populations, while simultaneous emphasis on maximum gene dispersion between subpopulations will further increase the chances of creating superior hybrids in terms of balanced tree vigour, yield and bean quality.
Selection for low pod index (number of pods required to produce 1 kg of cured beans) results in larger beans (and lower shell ratio) and helps reduce harvesting costs.
Consideration should be given to redesigning the tree architecture to improve photosynthetic efficiency and harvest, but also to increase the effectiveness of available resistance to diseases such as black pod and vascular-streak dieback (e.g. open canopy). This issue should be approached from both angles: genetic (vigour, branch habit, compact growth, tolerance of regular pruning) and agronomic (plant density, pruning).
Priorities in national cocoa breeding have shifted to host resistance to the globally important black pod and regionally important diseases, such as witches' broom in Brazil, swollen shoot virus in Ghana and vascular-streak dieback in South-East Asia. Disease resistance has to be a component of a fully integrated breeding programme, as resistance is only meaningful to the cocoa grower in combination with acceptable agronomic characteristics. In the case of vascular-streak dieback, high levels of host resistance have been found in some UA Forastero accessions (e.g. SCA and NA) and progress in breeding resistant hybrid cultivars is good. Host resistance to black pod exists in UA Forastero germplasm (e.g. SCA, PA and P) but levels are usually low, making it necessary to accumulate resistance genes in recombination crosses between progenitors prior to integration in the main breeding programme. Recent development of early preselection tests by artificial inoculation of leaf disks (Trinidad, Ivory Coast) should accelerate selection. An ecologically acceptable solution to the serious menace of the pod borer to the South-East Asian cocoa industry may be found in an integrated approach including biological control (e.g. egg parasitoids of the genus Trichogrammatoidea) and breeding for partial resistance or for a preventive mechanism, such as the presence of a sclerotic layer in the husk barring penetration of the borer in the pods of certain UA Forastero accessions.
There is evidence that in cocoa the genetic variance for components of all major agronomic characters is mainly due to additive gene effects. This should result in more efficient accumulation of favourable genes within breeding populations, while simultaneous emphasis on maximum gene dispersion between subpopulations will further increase the chances of creating superior hybrids in terms of balanced tree vigour, yield and bean quality.
Selection for low pod index (number of pods required to produce 1 kg of cured beans) results in larger beans (and lower shell ratio) and helps reduce harvesting costs.
Consideration should be given to redesigning the tree architecture to improve photosynthetic efficiency and harvest, but also to increase the effectiveness of available resistance to diseases such as black pod and vascular-streak dieback (e.g. open canopy). This issue should be approached from both angles: genetic (vigour, branch habit, compact growth, tolerance of regular pruning) and agronomic (plant density, pruning).
Prospects
Full exploitation of available cocoa genetic resources, by conventional and innovative breeding methods should lead to further improvement in yield and other desirable characteristics. Molecular marker technology is increasingly being applied in cocoa for germplasm management and to detect genetically divergent subpopulations. Recent experimental evidence also indicates that molecular marker-assisted selection has considerable potential for enhancing selection efficiency in cocoa, particularly with respect to host resistance to important diseases.
Indonesia remains the most competitive cocoa producer in the world because it has the lowest production costs. The only prospects for further expansion in Indonesia are smallholders in eastern Indonesia, but it remains to be seen whether the Sulawesi cocoa boom can be repeated on the other islands, and to what extent the cocoa pod borer will check further expansion. In Malaysia the cocoa pod borer and labour costs make cocoa a less interesting crop than oil palm and rubber, and large-scale conversion of cocoa to oil palm started in the early 1990s. In Papua New Guinea, large areas of suitable soils are available, but whether they will be planted to cocoa will depend on government support. In the Philippines, the main limitation to cocoa expansion is also the pod-borer. Vietnam could become the next country in the region for cocoa production because of the availability of large suitable areas and sufficient labour. The cocoa pod-borer is a very serious threat to the expansion of cocoa cultivation in South-East Asia. Strict quarantine measures must be observed, to prevent the insect from spreading into new areas.
For the long-term survival of the cocoa industry in South-East Asia it will also be essential to emphasize production systems that are sustainable and do not degrade the available natural resources. In that respect, studies on shade and nutritional requirements of cocoa in areas with inherently low soil fertility should have high priority.
Indonesia remains the most competitive cocoa producer in the world because it has the lowest production costs. The only prospects for further expansion in Indonesia are smallholders in eastern Indonesia, but it remains to be seen whether the Sulawesi cocoa boom can be repeated on the other islands, and to what extent the cocoa pod borer will check further expansion. In Malaysia the cocoa pod borer and labour costs make cocoa a less interesting crop than oil palm and rubber, and large-scale conversion of cocoa to oil palm started in the early 1990s. In Papua New Guinea, large areas of suitable soils are available, but whether they will be planted to cocoa will depend on government support. In the Philippines, the main limitation to cocoa expansion is also the pod-borer. Vietnam could become the next country in the region for cocoa production because of the availability of large suitable areas and sufficient labour. The cocoa pod-borer is a very serious threat to the expansion of cocoa cultivation in South-East Asia. Strict quarantine measures must be observed, to prevent the insect from spreading into new areas.
For the long-term survival of the cocoa industry in South-East Asia it will also be essential to emphasize production systems that are sustainable and do not degrade the available natural resources. In that respect, studies on shade and nutritional requirements of cocoa in areas with inherently low soil fertility should have high priority.
Literature
Akiyama, T. & Nishio, A., 1997. Sulawesi's cocoa boom: lessons of smallholder dynamism and a hands-off policy. Bulletin of Indonesian Economic Studies 33: 97—121.
Clapperton, J.F., 1994. A review of research to identify the origins of cocoa flavour characteristics. Cocoa Growers' Bulletin 48: 7—16.
Cuatrecasas, J., 1964. Cacao and its allies: a taxonomic revision of the genus Theobroma. Contributions from the United States National Herbarium 35: 379—614.
End, M.J., Wadsworth, R.M. & Hadley, P., 1992. International cocoa germplasm database. Biscuit, Cake, Chocolate & Confectionery Alliance (BCCCA) & The University of Reading, Reading, United Kingdom. Mimeographed Report. 355 pp.
Eskes, A.B. & Lanaud, C., 1997. Le cacaoyer [Cocoa]. In: Charrier, A., Jacquot, M., Hamon, S. & Nicolas, D. (Editors): L'amélioration des plantes tropicales [Tropical plant breeding]. Centre de Coopération Internationale en Recherche Agronomique pour le Développement (CIRAD) & Institut français de recherche scientifique pour le développement en coopération (ORSTOM), Montpellier, France. pp. 141—170.
6
Heijbroek, A.M.A. & Konijn, R.J., 1995. The cocoa and chocolate market. Rabobank, Utrecht, the Netherlands. 64 pp.
Keane, P.J. & Putter, C.A.J. (Editors), 1992. Cocoa pest and disease management in Southeast Asia and Australasia. FAO Plant Production and Protection Paper No 112. Food and Agriculture Organization of the United Nations, Rome, Italy. 223 pp.
Lass, R.A. & Wood, G.A.R. (Editors), 1985. Cocoa production: present constraints and priorities for research. World Bank Technical Paper No 39. World Bank, Washington, United States. 95 pp.
Lim, H.K.D. & Yeow, R., 1994. Development and management of plantation cocoa on scientific guidelines. In: Chee, K.H. (Editor): Management for enhanced profitability in plantations. Proceedings of the 1994 International Planters Conference on Management for Enhanced Profitability in Plantations, 24—26 October 1994, Kuala Lumpur, Malaysia. The Incorporated Society of Planters, Kuala Lumpur, Malaysia. pp. 177—190.
Mawardi, S., Winarno, H. & Suhendi, D., 1994. The present status of cocoa breeding at ICCRI: results and future programme. In: End, M.J., Eskes, A.B., Lee, M.T. & Lockwood, G. (Editors): Proceedings of the International Workshop on Cocoa Breeding Strategies, October 1994, Kuala Lumpur, Malaysia. Centre de Coopération Internationale en Recherche Agronomique pour le Développement (CIRAD) & International Group for Genetic Improvement of Cocoa (INGENIC), Montpellier, France. pp. 81—88.
Toxopeus, H., 1969. Cacao. In: Ferwerda, F.P. & Wit, F. (Editors): Outlines of perennial crop breeding in the tropics. Miscellaneous Papers No 4. Wageningen Agricultural University, Wageningen, the Netherlands. pp. 79—109.
12
Toxopeus, H. & Wessel, P.C. (Editors), 1983. Cocoa research in Indonesia 1900—1950. Archives of Cocoa Research. Vol. 2. American Cocoa Research Institute, Washington, United States & International Office of Cocoa and Chocolate, Brussels, Belgium. 293 pp.
Wood, G.A.R. & Lass, R.A. (Editors), 1985. Cocoa. 4th edition. Tropical Agriculture Series. Longman Scientific & Technical, Harlow, United Kingdom. 620 pp.
Clapperton, J.F., 1994. A review of research to identify the origins of cocoa flavour characteristics. Cocoa Growers' Bulletin 48: 7—16.
Cuatrecasas, J., 1964. Cacao and its allies: a taxonomic revision of the genus Theobroma. Contributions from the United States National Herbarium 35: 379—614.
End, M.J., Wadsworth, R.M. & Hadley, P., 1992. International cocoa germplasm database. Biscuit, Cake, Chocolate & Confectionery Alliance (BCCCA) & The University of Reading, Reading, United Kingdom. Mimeographed Report. 355 pp.
Eskes, A.B. & Lanaud, C., 1997. Le cacaoyer [Cocoa]. In: Charrier, A., Jacquot, M., Hamon, S. & Nicolas, D. (Editors): L'amélioration des plantes tropicales [Tropical plant breeding]. Centre de Coopération Internationale en Recherche Agronomique pour le Développement (CIRAD) & Institut français de recherche scientifique pour le développement en coopération (ORSTOM), Montpellier, France. pp. 141—170.
6
Heijbroek, A.M.A. & Konijn, R.J., 1995. The cocoa and chocolate market. Rabobank, Utrecht, the Netherlands. 64 pp.
Keane, P.J. & Putter, C.A.J. (Editors), 1992. Cocoa pest and disease management in Southeast Asia and Australasia. FAO Plant Production and Protection Paper No 112. Food and Agriculture Organization of the United Nations, Rome, Italy. 223 pp.
Lass, R.A. & Wood, G.A.R. (Editors), 1985. Cocoa production: present constraints and priorities for research. World Bank Technical Paper No 39. World Bank, Washington, United States. 95 pp.
Lim, H.K.D. & Yeow, R., 1994. Development and management of plantation cocoa on scientific guidelines. In: Chee, K.H. (Editor): Management for enhanced profitability in plantations. Proceedings of the 1994 International Planters Conference on Management for Enhanced Profitability in Plantations, 24—26 October 1994, Kuala Lumpur, Malaysia. The Incorporated Society of Planters, Kuala Lumpur, Malaysia. pp. 177—190.
Mawardi, S., Winarno, H. & Suhendi, D., 1994. The present status of cocoa breeding at ICCRI: results and future programme. In: End, M.J., Eskes, A.B., Lee, M.T. & Lockwood, G. (Editors): Proceedings of the International Workshop on Cocoa Breeding Strategies, October 1994, Kuala Lumpur, Malaysia. Centre de Coopération Internationale en Recherche Agronomique pour le Développement (CIRAD) & International Group for Genetic Improvement of Cocoa (INGENIC), Montpellier, France. pp. 81—88.
Toxopeus, H., 1969. Cacao. In: Ferwerda, F.P. & Wit, F. (Editors): Outlines of perennial crop breeding in the tropics. Miscellaneous Papers No 4. Wageningen Agricultural University, Wageningen, the Netherlands. pp. 79—109.
12
Toxopeus, H. & Wessel, P.C. (Editors), 1983. Cocoa research in Indonesia 1900—1950. Archives of Cocoa Research. Vol. 2. American Cocoa Research Institute, Washington, United States & International Office of Cocoa and Chocolate, Brussels, Belgium. 293 pp.
Wood, G.A.R. & Lass, R.A. (Editors), 1985. Cocoa. 4th edition. Tropical Agriculture Series. Longman Scientific & Technical, Harlow, United Kingdom. 620 pp.
Author(s)
M. Wessel & H. Toxopeus
Correct Citation of this Article
Wessel, M. and Toxopeus, H., 2000. Theobroma cacao L.. In: van der Vossen, H.A.M. and Wessel, M. (Editors): Plant Resources of South-East Asia No 16: Stimulants. PROSEA Foundation, Bogor, Indonesia. Database record: prota4u.org/prosea

All texts are licensed under a Creative Commons Attribution-Noncommercial-Share Alike 3.0 Netherlands License
This license does not include the illustrations (Maps,drawings,pictures); these remain all under copyright.