Record Number
66
PROSEA Handbook Number
12(1): Medicinal and poisonous plants 1
Taxon
Acorus calamus L.
Protologue
Sp. pl. 1: 324 (1753).
Family
ACORACEAE
Chromosome Numbers
2n = 24, 36, 48
Synonyms
Acorus terrestris Spreng. (1825), Acorus asiaticus Nakai (1936).
Vernacular Names
Sweet flag, sweet root, calamus (En). Calamus, acore odorant, acore vrai (Fr). Indonesia: daringo (Sundanese), dringo (Javanese), jerango (Sumatra). Malaysia: jerangau, deringu, jerangoh (Peninsular). Papua New Guinea: lepe (Angi, Enga), eseue (Mendi, Southern Highlands), wamala (Aroma, Central Province). Philippines: lubigan (Tagalog, Bisaya), acoro (Spanish), daraw (Iloko). Laos: hang khao nam. Thailand: kha chiang chee (northern), wan nam (central), haang khaao phaa (Chiang Mai). Vietnam: th[ur]y x[uw][ow]ng b[oof], x[uw][ow]ng b[oof], b[oof] b[oof] n[ees]p.
Origin and Geographic Distribution
Sweet flag is probably a native of China and India. Its use as a medicinal plant dates back to Egypt, Greek and Roman times. Sweet flag was distributed from its native range by rhizomes through trade and commerce, and arrived in Europe in the 16th Century. In the Malesian region, it is considered as naturalized and not truly wild. It is found in many parts of Indonesia, Malaysia, and Papua New Guinea and locally in the Philippines (Bontoc and Benguet Provinces), and outside Malesia in Indo-China and Thailand. It is also cultivated here and there.
Uses
The rhizomes of sweet flag have been used extensively in traditional medicine by Chinese, Indians, American Indians and others, and are still used in many regions. In Roman and Arabic civilizations aphrodisiac properties were attributed to the rhizome and it was used in North America and Europe as a panacea; in India, sweet flag has, for centuries, been an important medicinal aid for stomach complaints and colic in children. Since ancient times it has been reputed for its stimulant digestive virtues. In India, the rhizomes are traditionally used in an infusion to treat diarrhoea, dysentery, atonic dyspepsia and asthma, and for their carminative, expectorant, nauseant, antispasmodic, stomachic, vermifuge, sedative and emetic properties. In Vietnam, sweet flag is used to treat respiratory disorders (asthma, inflammation), rheumatism, remittent fevers, snake bites and as sedative. In Indonesia and Malaysia, the rhizomes are usually used externally to treat inflammation, rheumatism, lumbago and skin diseases, and internally after childbirth. In Java, sweet flag is an ingredient of certain 'jamus', and in the Philippines it is used as a masticatory against toothache and as a stimulant, carminative and antirheumatic. In Papua New Guinea, the leaves of sweet flag are taken as a tonic, and chewed to relieve tootache. The crushed rhizome is rubbed into the hair to kill lice. In Brunei, sweet flag is used to treat gastritis and diarrhoea and also as a poison antidote. In Thailand, the rhizomes are used as carminative, analgesic, anthelmintic, and to treat diarrhoea and dysentery. In Japan, Acorus oil is used as bathing agent, considered to be effective against skin diseases and to improve blood circulation. In the Unani (Greco-Arab) system of medicine, sweet flag is used to treat cardiovascular diseases. In Vietnam, a dose of 2-5 g/day is administered in decoction. In modern phytotherapy, sweet flag rhizomes ('calami rhizoma') can, on the basis of their constituents, be called a bitter aromatic. This is principally used as a stomachic and carminative (internally) and externally as a rubefacient and in the treatment of seborrhoea (as a bath).
The fragrant oil obtained from the rhizome is not only used medicinally, but also for flavouring alcoholic beverages (e.g. vermouths), fish, sweets and cakes, in perfumes and sacred oils and as an insecticide. As an insecticide, it is often used as emulsified foliage spray. The use of rhizome powder in warehoand on the farm to protect stored grain, rice and pulses from insect pests has proved fairly effective and economical; powdered rhizomes may also reduce the extent of fungal and bacterial contamination. The hydroalcoholic extract is important in food technology, whereas the essential oil is important in perfumery. The extract is also useful as an antibacterial and antifungal agent. In ancient times the fragrant leaves were used as a strewing herb to remove disagreeable odours and to deter pests. The candied rhizome was a confection in Europe and America. Sweet flag is used in magic rituals in New Guinea and it was also used in snuff rituals by American Indians.
The fragrant oil obtained from the rhizome is not only used medicinally, but also for flavouring alcoholic beverages (e.g. vermouths), fish, sweets and cakes, in perfumes and sacred oils and as an insecticide. As an insecticide, it is often used as emulsified foliage spray. The use of rhizome powder in warehoand on the farm to protect stored grain, rice and pulses from insect pests has proved fairly effective and economical; powdered rhizomes may also reduce the extent of fungal and bacterial contamination. The hydroalcoholic extract is important in food technology, whereas the essential oil is important in perfumery. The extract is also useful as an antibacterial and antifungal agent. In ancient times the fragrant leaves were used as a strewing herb to remove disagreeable odours and to deter pests. The candied rhizome was a confection in Europe and America. Sweet flag is used in magic rituals in New Guinea and it was also used in snuff rituals by American Indians.
Production and International Trade
The dried rhizomes of sweet flag are traded locally on markets. Nowadays this trade is not very important, but the extremely large area of distribution resulting from former cultivation indicates that it must have been considerable in the past. The oil is traded in somewhat larger amounts in Europe, mainly for flavouring alcoholic drinks. It is reported that annually about 200 t of rhizomes are used for manufacturing medicines in the Ukraine, and about 20 t in Germany. Almost 30 medicinal preparations which contain sweet flag are available in Europe.
Properties
The rhizome of sweet flag is aromatic, smelling of citrus, with a bitter spicy taste. The rhizome contains 2-6(-9)% of a pale yellow to pale brown essential oil with a woody spicy odour with increasingly sweet afternotes and great tenacity. It is normally obtained by steam distillation of fresh or dried unpeeled rhizomes.
Thanks to the great amount of research done on the chemical compounds in the rhizome it is known that sweet flag oil is a source of oxygenated sesquiterpenes of great structural variety. The major chemical constituents of the essential oil are phenylpropanes, monoterpenes and thermolabile sesquiterpenoids. As many as 250 or so volatile components have been detected in the oil of the triploid European var. calamus, and about 100 in the tetraploid var. angustatus. The major constituents include 'BETA'-asarone (cis-isoasarone), methyleugenol, cis-methylisoeugenol, geranylacetate, 'BETA'-farnesene, shyobunone, epishyobunone, isoshyobunone, calamusenone and acorenone. The proportion of each chemical component in the oil varies among the varieties, depending on the degree of polyploidy. The concentration of 'BETA'-asarone varies markedly; it may form as much as 4-8% of the rhizome and up to 96% of the essential oil in tetraploid Asiatic plants, but only about 0.3% of the rhizome and up to 5% of the oil in triploid European plants, but is absent (or undetectable) in diploid North American plants. The asarone is odourless, so the minor components are decisive in the fragrance of the oil.
The 2 stereoisomers 'ALFA'-asarone (trans-isoasarone) and 'BETA'-asarone (cis-isoasarone) are reported to have psychoactive effects. This has been attributed to the structure, which is similar to that of amphetamines and of the hallucinogenic compound mescaline. Asarone has a relaxing effect on smooth muscle tissue, and the oil has been found to induce spasmolytic activity in rabbit intestines, aortae and uteri. Experiments with guinea-pig ilia have demonstrated that the cortex of the rhizome acts as an antispasmodic agent. In tests on laboratory animals it also showed antihistamine, anticonvulsant and antipyretic activity. It has also been found to act as neuroleptic enhancer, central nervous system depressant, carcinogen, hypothermic, hypotensive, analgesic, anti-inflammatory, bronchodilator, respiration inhibitor, hepatotoxin and antifibrillatory. However, other tests have shown negative results for many of these activities. Although 'BETA'-asarone is reported to relax smooth muscle tissue, the American drug that does not contain this component has also been shown to be spasmolytically active. These results suggest that 'BETA'-asarone cannot be solely responsible for the effect and that other antispasmodic compounds must also be present.
The oil is reported effective for hypotensive relief in cats and as an anticonvulsant in pregnant mice. An oral dose of 500 mg/kg of the ethanolic extract showed significant anti-secretory and anti-ulcerogenic activity in rats subjected to pyloric ligation, reserpine and cysteamine administration, and had a highly significant protective effect against cytodestructive agents; these results support the use of sweet flag for the treatment of gastropathy in traditional medicine. Extracts have shown effective antifungal and antibacterial activity, and are reported to be effective against leeches. The rhizome has shown insecticidal activity against a wide range of insect species. Both antifeedant activity and contact toxicity have been reported, and the oil can cause sterility in some insects.
The oil has shown anti-amoebic activity against Paramecium caudatum and nematicidal activity against Ascaris lumbricoides, Toxocara canis and Meloidogyne incognita, as well as acaricidal against the tick Boophilus microplus. It has also been found to inhibit the germination of weeds in cotton. Tests with ground rhizomes mixed with cotton seeds showed promising results for the use as seed protectant against the fungus Sclerotium rolfsii that causes damping-off disease. The active compound seems to be 'BETA'-asarone which has toxic and sterilizing effects. Since the amount of 'BETA'-asarone is highly dependent on the source of the plant material, care should be taken to carefully record this source. Dried rhizomes have been found to exhibit no antiviral and antitumour activity.
Tannins, starches, mucin, soft gums and resins are also present. Rhizomes contain approximately 10% moisture, 8% sugar, 16% protein, 2% nitrogen, 6.5% ash and 28% ethanol-soluble extractive. Under certain conditions sweet flag is poisonous, causing disturbed digestion, gastro-enteritis and persistent constipation, followed by diarrhoea and passage of blood into the faeces. The use of sweet flag is prohibited in the United States and Canada, because cancerous tumours were found in laboratory animals treated with sweet flag for long periods. The carcinogenic agent seems to be 'BETA'-asarone, from which mutagenic (demonstrated on Salmonella typhimurium) and chromosome damaging have also been reported. In general the diploid (North American) variety void of 'BETA'-asarone should be used for pharmaceutical applications. However, triploid European forms poor in 'BETA'-asarone (< 0.5%) are acceptable, providing they are not used for prolonged periods. The recommended limit in Europe for flavouring is 0.1 mg/kg in foods and 1 mg/kg in alcoholic beverages and spice mixtures. A rapid and reliable thin-layer chromatographic method, allowing determination down to 0.01 mg/l is available. In some cases, the oil has been known to cause dermatitis when in contact with the skin.
The properties of the mainland Asiatic Acorus gramineus have been studied much less than those of Acorus calamus. It seems to contain less essential oil, but this oil has a high concentration of 'BETA'-asarone (63-81%), whereas 'ALFA'-asarone has also been reported as one of the important principles of the dry rhizome. The hexane fraction from methanolic extracts revealed potent inhibitory activity against the resistance of multi-drug resistant Staphylococcus aureus; benzoic acid phenylmethyl ster (benzyl benzoate) has been identified as active principle. A water extract of the dry rhizome decreased the locomotor activity of mice and increased the pentobarbital-induced sleeping time, in a dose dependent way.
Thanks to the great amount of research done on the chemical compounds in the rhizome it is known that sweet flag oil is a source of oxygenated sesquiterpenes of great structural variety. The major chemical constituents of the essential oil are phenylpropanes, monoterpenes and thermolabile sesquiterpenoids. As many as 250 or so volatile components have been detected in the oil of the triploid European var. calamus, and about 100 in the tetraploid var. angustatus. The major constituents include 'BETA'-asarone (cis-isoasarone), methyleugenol, cis-methylisoeugenol, geranylacetate, 'BETA'-farnesene, shyobunone, epishyobunone, isoshyobunone, calamusenone and acorenone. The proportion of each chemical component in the oil varies among the varieties, depending on the degree of polyploidy. The concentration of 'BETA'-asarone varies markedly; it may form as much as 4-8% of the rhizome and up to 96% of the essential oil in tetraploid Asiatic plants, but only about 0.3% of the rhizome and up to 5% of the oil in triploid European plants, but is absent (or undetectable) in diploid North American plants. The asarone is odourless, so the minor components are decisive in the fragrance of the oil.
The 2 stereoisomers 'ALFA'-asarone (trans-isoasarone) and 'BETA'-asarone (cis-isoasarone) are reported to have psychoactive effects. This has been attributed to the structure, which is similar to that of amphetamines and of the hallucinogenic compound mescaline. Asarone has a relaxing effect on smooth muscle tissue, and the oil has been found to induce spasmolytic activity in rabbit intestines, aortae and uteri. Experiments with guinea-pig ilia have demonstrated that the cortex of the rhizome acts as an antispasmodic agent. In tests on laboratory animals it also showed antihistamine, anticonvulsant and antipyretic activity. It has also been found to act as neuroleptic enhancer, central nervous system depressant, carcinogen, hypothermic, hypotensive, analgesic, anti-inflammatory, bronchodilator, respiration inhibitor, hepatotoxin and antifibrillatory. However, other tests have shown negative results for many of these activities. Although 'BETA'-asarone is reported to relax smooth muscle tissue, the American drug that does not contain this component has also been shown to be spasmolytically active. These results suggest that 'BETA'-asarone cannot be solely responsible for the effect and that other antispasmodic compounds must also be present.
The oil is reported effective for hypotensive relief in cats and as an anticonvulsant in pregnant mice. An oral dose of 500 mg/kg of the ethanolic extract showed significant anti-secretory and anti-ulcerogenic activity in rats subjected to pyloric ligation, reserpine and cysteamine administration, and had a highly significant protective effect against cytodestructive agents; these results support the use of sweet flag for the treatment of gastropathy in traditional medicine. Extracts have shown effective antifungal and antibacterial activity, and are reported to be effective against leeches. The rhizome has shown insecticidal activity against a wide range of insect species. Both antifeedant activity and contact toxicity have been reported, and the oil can cause sterility in some insects.
The oil has shown anti-amoebic activity against Paramecium caudatum and nematicidal activity against Ascaris lumbricoides, Toxocara canis and Meloidogyne incognita, as well as acaricidal against the tick Boophilus microplus. It has also been found to inhibit the germination of weeds in cotton. Tests with ground rhizomes mixed with cotton seeds showed promising results for the use as seed protectant against the fungus Sclerotium rolfsii that causes damping-off disease. The active compound seems to be 'BETA'-asarone which has toxic and sterilizing effects. Since the amount of 'BETA'-asarone is highly dependent on the source of the plant material, care should be taken to carefully record this source. Dried rhizomes have been found to exhibit no antiviral and antitumour activity.
Tannins, starches, mucin, soft gums and resins are also present. Rhizomes contain approximately 10% moisture, 8% sugar, 16% protein, 2% nitrogen, 6.5% ash and 28% ethanol-soluble extractive. Under certain conditions sweet flag is poisonous, causing disturbed digestion, gastro-enteritis and persistent constipation, followed by diarrhoea and passage of blood into the faeces. The use of sweet flag is prohibited in the United States and Canada, because cancerous tumours were found in laboratory animals treated with sweet flag for long periods. The carcinogenic agent seems to be 'BETA'-asarone, from which mutagenic (demonstrated on Salmonella typhimurium) and chromosome damaging have also been reported. In general the diploid (North American) variety void of 'BETA'-asarone should be used for pharmaceutical applications. However, triploid European forms poor in 'BETA'-asarone (< 0.5%) are acceptable, providing they are not used for prolonged periods. The recommended limit in Europe for flavouring is 0.1 mg/kg in foods and 1 mg/kg in alcoholic beverages and spice mixtures. A rapid and reliable thin-layer chromatographic method, allowing determination down to 0.01 mg/l is available. In some cases, the oil has been known to cause dermatitis when in contact with the skin.
The properties of the mainland Asiatic Acorus gramineus have been studied much less than those of Acorus calamus. It seems to contain less essential oil, but this oil has a high concentration of 'BETA'-asarone (63-81%), whereas 'ALFA'-asarone has also been reported as one of the important principles of the dry rhizome. The hexane fraction from methanolic extracts revealed potent inhibitory activity against the resistance of multi-drug resistant Staphylococcus aureus; benzoic acid phenylmethyl ster (benzyl benzoate) has been identified as active principle. A water extract of the dry rhizome decreased the locomotor activity of mice and increased the pentobarbital-induced sleeping time, in a dose dependent way.
Adulterations and Substitutes
In India, the rhizomes of Alpinia galanga (L.) Willd. and Alpinia officinarum Hance are commonly used as adulterant for medicinal purposes. Neem seed oil (from Azadirachta indica A.H.L. Juss.) has similar insecticidal properties, as do extracts from leaves and fruits of Melia azedarach L., from the rhizome of turmeric (Curcuma longa L.) and from garlic (Allium sativum L.), and the oils from basil (Ocimum spp.), star anise (Illicium verum Hook.f.) and nutmeg (Myristica fragrans Houtt.).
Description
A perennial glabrous herb up to 150 cm tall; rhizome creeping, extensively branched, up to 3 cm in diameter, pale yellowish to pinkish-brown outside, whitish, sometimes slightly pinkish inside, upper surface marked with large V-shaped leaf-scars, longitudinally furrowed, under surface with circular pitted scars of rootlets arranged in irregular lines. Leaves erect, linear-ensiform, with obliquely acuminate apex, often characteristically corrugated at one side in the upper part, with distinct midrib and numerous thin parallel veins, glossy green but often reddish towards base, aromatic. Inflorescence arising from the rhizome, erect, with a cylindrical, straight or slightly curved spadix up to 10 cm long and produced from about the middle of an apparent leaf consisting of the compressed trigonous leaf-like peduncle and the leaf-like spathe forming a continuation of the peduncle. Flowers densely arranged on the spadix, bisexual, 3-merous; tepals 6, in 2 whorls, free, narrowly oblong, 2-3 mm long; stamens 6, free, about 3 mm long, with strap-shaped filaments and orbicular-elliptical anthers dehiscent by a longitudinal slit; ovary superior, subquadrangular, 2-3-celled, stigma sessile, subconical. Fruit a 2-3-celled berry, turbinate and prismatic with pyramidal top, few-seeded, reddish. Seeds ellipsoid.
Image
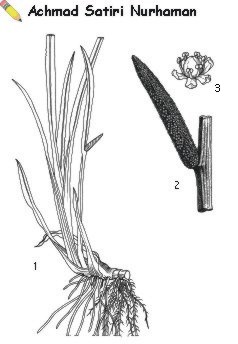 | Acorus calamus L. — 1, plant habit; 2, inflorescence; 3, flower. |
Growth and Development
Rhizomes of sweet flag can rapidly develop leaves and inflorescences under favourable conditions, e.g. in spring in temperate climates. Rhizomes show a remarkable tolerance of anaerobic conditions. Plants are usually exposed to periods of flooding and consequently to anaerobic conditions, and can survive for about 2 months in the complete absence of oxygen. The physiology of the plant is adapted to these conditions: the expression of genes enclosing glycolytic enzymes is induced during periods of submergence.
In certain populations plants often do not flower for years. In Malesia, sweet flag is even reported to flower rarely. In many areas the plant does not develop fruits. In Java, the tetraploid plants do fruit sometimes.
In certain populations plants often do not flower for years. In Malesia, sweet flag is even reported to flower rarely. In many areas the plant does not develop fruits. In Java, the tetraploid plants do fruit sometimes.
Other Botanical Information
Acorus has traditionally been placed in the family Araceae, where it is included in the subfamily Pothoideae together with e.g. Pothos and Anthurium and forms the tribe Acoreae. However, recent taxonomic studies suggest that on the basis of morphological, anatomical, developmental and molecular evidence the genus should be placed in the monotypic family Acoraceae. The recent suggestion that Acorus is a member of the oldest extant lineage of monocotyledons is based on phylogenetic analysis from DNA sequences; there is supporting morphological, anatomical and embryological evidence. Acorus is generally considered to consist of 2 species, but a third species has been distinguished in China.
Acorus calamus is highly variable in many respects. The size and shape of rhizomes, leaves and spadices are greatly affected by growth conditions. The species has been subdivided primarily on the basis of genome differences. Var. americanus (Raf.) Wulff is diploid and fertile and occurs from North America to Siberia, var. calamus is triploid and sterile and occurs in Europe, the Himalayas and temperate India and certain parts of the United States of America, and var. angustatus Bess. is tetraploid and partly fertile and occurs in eastern and southern Asia from Japan and China to the Malesian region. The tropical ecotype of this last variety is sometimes called var. verus L.
Another polytypic species, Acorus gramineus Soland. ex Aiton, is diploid. It differs from Acorus calamus in its usually very narrow worm-like spadix, leaves without distinct midrib but with ribs on the margins, and tougher rhizomes and leaves. It is native to mainland south-eastern Asia (from India, Thailand and Indo-China to China) and Japan, and is used there for similar purposes as Acorus calamus.
Acorus calamus is highly variable in many respects. The size and shape of rhizomes, leaves and spadices are greatly affected by growth conditions. The species has been subdivided primarily on the basis of genome differences. Var. americanus (Raf.) Wulff is diploid and fertile and occurs from North America to Siberia, var. calamus is triploid and sterile and occurs in Europe, the Himalayas and temperate India and certain parts of the United States of America, and var. angustatus Bess. is tetraploid and partly fertile and occurs in eastern and southern Asia from Japan and China to the Malesian region. The tropical ecotype of this last variety is sometimes called var. verus L.
Another polytypic species, Acorus gramineus Soland. ex Aiton, is diploid. It differs from Acorus calamus in its usually very narrow worm-like spadix, leaves without distinct midrib but with ribs on the margins, and tougher rhizomes and leaves. It is native to mainland south-eastern Asia (from India, Thailand and Indo-China to China) and Japan, and is used there for similar purposes as Acorus calamus.
Ecology
Sweet flag is a component of semi-aquatic habitats, usually in eutrophic locations. It can be a vigorous invader of new sites. In Malesia, it is found along ditches, pools, fish-ponds and marshes, and is sometimes cultivated. In Java, it is found up to 2100 m altitude. Sweet flag can be planted on clayey loams and light alluvial soils.
Propagation and planting
Sweet flag can be propagated easily from pieces of rhizome. The field is ploughed and watered prior to planting, and sometimes green manure is incorporated. The rhizome pieces to be planted are generally 6 cm long and have growing tops. They are planted at 20 cm x 20 cm. Roots start to develop 10-15 days after planting, and are soon followed by leaves. In India, sweet flag has been successfully intercropped with poplar (Populus sp.).
Harvesting
Sweet flag can be harvested within one year after planting. The timing strongly affects the yield of essential oil. In temperate climates, the best period for harvesting is autumn, and the least suitable period is spring.
Yield
In India, the average weight of individual green rhizomes harvested 10 months after planting was 175 g (95 g after drying). They contained 1.4% essential oil on average, and the highest yield was 10.4 kg/ha.
Handling After Harvest
After harvesting, the rhizomes are freed from leaves and roots and washed; they are dried unpeeled, cut in pieces and sold on the market. Storing powdered rhizomes for 2 months at 29°C and 65-75% relative humidity did not reduce their effectiveness as an insecticide.
Genetic Resources and Breeding
Genetic resources and breeding Sweet flag has a very large area of distribution and is common in many parts of the world in habitats which are not at risk of destruction. However, locally (e.g. in certain parts of India) it is endangered or even on the verge of extinction. The great genetic variability which is correlated with differences in chemical composition should be taken into account when making germplasm collections and when breeding for special purposes.
Prospects
Research findings suggest that sweet flag may have applications for several ailments for which it has a historical record of use. It may still have beneficial applications in modern medicine. Its use in perfumes, foods and beverages is limited because of the carcinogenic phenylpropane derivative 'BETA'-asarone present in the extract, but the presence of diploid populations which seem to be free from this component offers new opportunities for more extensive use in the future after selection. Sweet flag might have good for commercial exploitation as a pesticide of plant origin.
Literature
Bruneton, J., 1995. Pharmacognosy, phytochemistry, medicinal plants. Lavoisier Publishing, Paris, France. pp. 463-464.
Dey, D. & Das, M.N., 1982. Pharmacognostic studies of Acorus calamus and its adulterants. Acta Botanica Indica 10(1): 28-35.
Grayum, M.H., 1987. A summary of evidence and arguments supporting the removal of Acorus from the Araceae. Taxon 36(4): 723-729.
Mazza, G., 1985. Gas chromatographic and mass spectrometric studies of the constituents of the rhizome of calamus. Journal of Chromatography 328: 179-206.
Motley, T.J., 1994. The ethnobotany of sweet flag, Acorus calamus (Araceae). Economic Botany 48(4): 397-412.
Phillip, J., Nair, G.S., Premalatha & Sudhadevi, P.K., 1992. Effect of planting materials and time of harvest on yield and essential oil content of rhizomes in Acorus calamus. Indian Cocoa, Arecanut and Spices Journal 16(2): 63-65.
Rafatullah, S., Tariq, M., Mossa, J.S., Al-Yahya, M.A., Al-Said, M.S. & Ageel, A.M., 1994. Anti-secretagogue, anti-ulcer and cytoprotective properties of Acorus calamus in rats. Fitoterapia 65(1): 19-23.
Röst, L.C.M., 1979. Biosystematic investigations with Acorus. 4. Communication. A synthetic approach to the classification of the genus. Planta Medica 37(4): 289-307. Röst, L.C.M. & Bos, R., 1979. Biosystematic investigations with Acorus L. 3. Communication. Constituents of essential oils. Planta Medica 36(4): 350-361.
Stahl, E. & Keller, K., 1981. Zur Klassifizierung handelsüblicher Kalmusdrogen [About the classification of commercial Acorus calamus drugs]. Planta Medica 43(2): 128-140.
Dey, D. & Das, M.N., 1982. Pharmacognostic studies of Acorus calamus and its adulterants. Acta Botanica Indica 10(1): 28-35.
Grayum, M.H., 1987. A summary of evidence and arguments supporting the removal of Acorus from the Araceae. Taxon 36(4): 723-729.
Mazza, G., 1985. Gas chromatographic and mass spectrometric studies of the constituents of the rhizome of calamus. Journal of Chromatography 328: 179-206.
Motley, T.J., 1994. The ethnobotany of sweet flag, Acorus calamus (Araceae). Economic Botany 48(4): 397-412.
Phillip, J., Nair, G.S., Premalatha & Sudhadevi, P.K., 1992. Effect of planting materials and time of harvest on yield and essential oil content of rhizomes in Acorus calamus. Indian Cocoa, Arecanut and Spices Journal 16(2): 63-65.
Rafatullah, S., Tariq, M., Mossa, J.S., Al-Yahya, M.A., Al-Said, M.S. & Ageel, A.M., 1994. Anti-secretagogue, anti-ulcer and cytoprotective properties of Acorus calamus in rats. Fitoterapia 65(1): 19-23.
Röst, L.C.M., 1979. Biosystematic investigations with Acorus. 4. Communication. A synthetic approach to the classification of the genus. Planta Medica 37(4): 289-307. Röst, L.C.M. & Bos, R., 1979. Biosystematic investigations with Acorus L. 3. Communication. Constituents of essential oils. Planta Medica 36(4): 350-361.
Stahl, E. & Keller, K., 1981. Zur Klassifizierung handelsüblicher Kalmusdrogen [About the classification of commercial Acorus calamus drugs]. Planta Medica 43(2): 128-140.
Other Selected Sources
[97] Backer, C.A. & Bakhuizen van den Brink Jr, R.C., 1963-1968. Flora of Java. 3 volumes. Noordhoff, Groningen, the Netherlands. Vol. 1 (1963) 647 pp., Vol. 2 (1965) 641 pp., Vol. 3 (1968) 761 pp.
[190] Brown, W.H., 1951-1957. Useful plants of the Philippines. Reprint of the 1941-1943 edition. 3 volumes. Technical Bulletin 10. Department of Agriculture and Natural Resources. Bureau of Printing, Manila, the Philippines. Vol. 1 (1951) 590 pp., Vol. 2 (1954) 513 pp., Vol. 3 (1957) 507 pp.
[194] Bucher, M., Brandle, R. & Kuhlemeier, C., 1996. Glycolytic gene expression in amphibious Acorus calamus L. under natural conditions. Plant and Soil 178(1): 75-82.
[202] Burkill, I.H., 1966. A dictionary of the economic products of the Malay Peninsula. Revised reprint. 2 volumes. Ministry of Agriculture and Co-operatives, Kuala Lumpur, Malaysia. Vol. 1 (A-H) pp. 1-1240. Vol. 2 (I-Z) pp. 1241-2444.
[297] Currò, P., Micali, G. & Lanuzza, F., 1987. Determination of 'BETA'-asarone, safrole, isosafrole and anethole in alcoholic drinks by high-performance liquid chromatography. Journal of Chromatography 404: 273-278.
[332] de Padua, L.S., Lugod, G.C. & Pancho, J.V., 1977-1983. Handbook on Philippine medicinal plants. 4 volumes. Documentation and Information Section, Office of the Director of Research, University of the Philippines at Los Baños, the Philippines.
[348] Dey, D. & Das, M.N., 1988. Pharmacognosy of antidysenteric drugs of Indian medicine. Acta Botanica Indica 16(2): 216-226.
[350] Dharma, A.P., 1981. Indonesische geneeskrachtige planten [Indonesian medicinal plants]. De Driehoek, Amsterdam, the Netherlands. 168 pp.
[386] Duvall, M.R., Learn, G.H., Eguiarte, L.E. & Clegg, M.T., 1993. Phylogenetic analysis of rbcL sequences identifies Acorus calamus as the primal extant monocotyledon. Proceedings of the National Academy of Sciences of the United States of America 90(10): 4641-4644.
[580] Heyne, K., 1950. De nuttige planten van Indonesië [The useful plants of Indonesia]. 3rd Edition. 2 volumes. W. van Hoeve, 's-Gravenhage, the Netherlands/Bandung, Indonesia. 1660 + CCXLI pp.
[597] Holdsworth, D.K., 1977. Medicinal plants of Papua New Guinea. Technical Paper No 175. South Pacific Commission, Noumea, New Caledonia. 123 pp.
[964] Mohiddin, M.Y., Wong Chin & Holdsworth, D.K., 1991. Traditional medicinal plants of Brunei Darussalam. Part II. Sengkurong. International Journal of Pharmacognosy 29(4): 252-258.
[1035] Nguyen Van Duong, 1993. Medicinal plants of Vietnam, Cambodia and Laos. Mekong Printing, Santa Ana, California, United States. 528 pp.
[1178] Quisumbing, E., 1978. Medicinal plants of the Philippines. Katha Publishing Co., Quezon City, the Philippines. 1262 pp.
[1312] Shah, S.C. & Gupta, L.K., 1977. Note on pharmacognostic study of the rhizome of Acorus calamus Linn. Indian Journal of Agricultural Research 11(2): 113-115.
[1566] Wichtl, M. (Editor), 1994. Herbal drugs and phytopharmaceuticals. CRC Press, Boca Raton, Florida, United States. 800 pp.
[190] Brown, W.H., 1951-1957. Useful plants of the Philippines. Reprint of the 1941-1943 edition. 3 volumes. Technical Bulletin 10. Department of Agriculture and Natural Resources. Bureau of Printing, Manila, the Philippines. Vol. 1 (1951) 590 pp., Vol. 2 (1954) 513 pp., Vol. 3 (1957) 507 pp.
[194] Bucher, M., Brandle, R. & Kuhlemeier, C., 1996. Glycolytic gene expression in amphibious Acorus calamus L. under natural conditions. Plant and Soil 178(1): 75-82.
[202] Burkill, I.H., 1966. A dictionary of the economic products of the Malay Peninsula. Revised reprint. 2 volumes. Ministry of Agriculture and Co-operatives, Kuala Lumpur, Malaysia. Vol. 1 (A-H) pp. 1-1240. Vol. 2 (I-Z) pp. 1241-2444.
[297] Currò, P., Micali, G. & Lanuzza, F., 1987. Determination of 'BETA'-asarone, safrole, isosafrole and anethole in alcoholic drinks by high-performance liquid chromatography. Journal of Chromatography 404: 273-278.
[332] de Padua, L.S., Lugod, G.C. & Pancho, J.V., 1977-1983. Handbook on Philippine medicinal plants. 4 volumes. Documentation and Information Section, Office of the Director of Research, University of the Philippines at Los Baños, the Philippines.
[348] Dey, D. & Das, M.N., 1988. Pharmacognosy of antidysenteric drugs of Indian medicine. Acta Botanica Indica 16(2): 216-226.
[350] Dharma, A.P., 1981. Indonesische geneeskrachtige planten [Indonesian medicinal plants]. De Driehoek, Amsterdam, the Netherlands. 168 pp.
[386] Duvall, M.R., Learn, G.H., Eguiarte, L.E. & Clegg, M.T., 1993. Phylogenetic analysis of rbcL sequences identifies Acorus calamus as the primal extant monocotyledon. Proceedings of the National Academy of Sciences of the United States of America 90(10): 4641-4644.
[580] Heyne, K., 1950. De nuttige planten van Indonesië [The useful plants of Indonesia]. 3rd Edition. 2 volumes. W. van Hoeve, 's-Gravenhage, the Netherlands/Bandung, Indonesia. 1660 + CCXLI pp.
[597] Holdsworth, D.K., 1977. Medicinal plants of Papua New Guinea. Technical Paper No 175. South Pacific Commission, Noumea, New Caledonia. 123 pp.
[964] Mohiddin, M.Y., Wong Chin & Holdsworth, D.K., 1991. Traditional medicinal plants of Brunei Darussalam. Part II. Sengkurong. International Journal of Pharmacognosy 29(4): 252-258.
[1035] Nguyen Van Duong, 1993. Medicinal plants of Vietnam, Cambodia and Laos. Mekong Printing, Santa Ana, California, United States. 528 pp.
[1178] Quisumbing, E., 1978. Medicinal plants of the Philippines. Katha Publishing Co., Quezon City, the Philippines. 1262 pp.
[1312] Shah, S.C. & Gupta, L.K., 1977. Note on pharmacognostic study of the rhizome of Acorus calamus Linn. Indian Journal of Agricultural Research 11(2): 113-115.
[1566] Wichtl, M. (Editor), 1994. Herbal drugs and phytopharmaceuticals. CRC Press, Boca Raton, Florida, United States. 800 pp.
Author(s)
Nguyen Van Dzu
Correct Citation of this Article
Nguyen van Dzu, 1999. Acorus calamus L.. In: de Padua, L.S., Bunyapraphatsara, N. and Lemmens, R.H.M.J. (Editors): Plant Resources of South-East Asia No 12(1): Medicinal and poisonous plants 1. PROSEA Foundation, Bogor, Indonesia. Database record: prota4u.org/prosea

All texts are licensed under a Creative Commons Attribution-Noncommercial-Share Alike 3.0 Netherlands License
This license does not include the illustrations (Maps,drawings,pictures); these remain all under copyright.