Record Number
829
PROSEA Handbook Number
14: Vegetable oils and fats
Taxon
Cocos nucifera L.
Protologue
Sp. pl.: 1188 (1753).
Family
PALMAE
Chromosome Numbers
2n = 32
Synonyms
Cocos nana Griff. (1851).
Vernacular Names
Coconut (palm and fruit) (En). Cocotier (palm), coco (fruit) (Fr). Indonesia: kelapa (general), nyiur (Malay), krambil (Javanese). Malaysia: kelapa. Papua New Guinea: kokonas. Philippines: niyog (Pilipino, Tagalog), iniug (Ibanag), lubi, ungut (Bisaya), laying (Manobo). Burma (Myanmar): ong. Cambodia: doong. Laos: phaawz. Thailand: ma phrao (general), kho-saa (Karen-Mae Hong Son), dung (Chong-Chanthaburi). Vietnam: d[uwf]a.
Origin and Geographic Distribution
Cocos nucifera is native to the coastal regions of tropical Asia and the Pacific, but its primary centre of origin is the subject of speculation. Fossil coconuts have been found as far apart as India and New Zealand. The ability of the thickly husked and slow germinating fruit of wild coconut (called Niu Kafa type) to remain viable after floating long distances at sea ensured wide natural dispersal in the Indo-Pacific long before domestication may have started in Malesia. The domesticated coconut (called Niu Vai type) has a robust stem and large fruits, which however cannot survive long periods of floating at sea because of thinner husks and shells and quicker germination. Initial dissemination of the domesticated coconut coincided with migrations of Malesian peoples to the Pacific and India, which started some 3000 years ago. Where wild coconut already occurred, there was opportunity for introgression with domesticated types, as both retained full crosscompatibility. Polynesian, Malay and Arab navigators played an important role in further dispersal of coconut into the Pacific, Asia and East Africa. The coconut became truly pantropical in the 16th Century after European explorers had taken it to West Africa, the Caribbean and the Atlantic coast of tropical America.
Uses
The coconut palm has been called the 'tree of life', the 'tree of heaven' and 'one of nature=s greatest gifts to man' because of its value as provider of so may useful products. For domestic oil extraction the fresh endocarp of mature fruits is grated and squeezed with hot water; for industrial production the endosperm is dried to copra and taken to the mill for oil extraction. High-grade oil is used for cooking or in the manufacture of margarine, shortening, filled milk, ice-cream and confectioneries. Oil of lower grades is processed into soap, detergents, cosmetics, shampoos, paints, varnishes and pharmaceutical products. Remnant fatty acids and alcohols and methyl esters find application as components of emulsifiers and surfactants. The presscake or copra meal is a good feed.
Coconut milk or cream (Indonesia/Malaysia: 'santan'; Philippines: 'gata') pressed from freshly grated endosperm mixed with water has been a traditional ingredient in many Asian food and bakery products. It is now also marketed in pasteurized and homogenized canned or powdered form. After preparing coconut milk by boiling grated fresh coconut meat a presscake remains. In Java it is considered a delicacy, named 'blondo' or 'galendo'. Skimmed milk powder, produced after boiling fresh coconut milk and removing the floating oil, contains 25% hydrolyzed starch and can be mixed with water to make a beverage. Protein can be separated by ultrafiltration and spray-dried into a white powder, which is very suitable for infant nutrition. Coconut skim milk is an essential ingredient of a gelatinous delicacy ('nata de coco') in the Philippines used in sweetened desserts, ice-cream and confectionery. Shredded or thinly sliced and desiccated fresh coconut endosperm is a favourite side-dish and ingredient in many confectionery, bakery and snack food products. Ball copra is an Indian speciality produced by slow drying, dehusking and shelling of the whole mature nut. It is used to prepare sweets offered during religious and cultural rites and in traditional medicine.
The nut cavity is filled with water that tastes sweet when the coconut is young. Coconut water is now commercially preserved without altering its typical flavour. It can also be used in the production of 'nata de coco', which is a gelatinous dessert produced by the action of bacteria on coconut water or diluted coconut milk, developed in the Philippines. It is a source of inexpensive growth hormone products for horticulture, such as the Cocogro developed in the Philippines. The tender, jelly-like endosperm of young coconuts is a delicacy consumed directly or grated and mixed with food. The haustorium or apple of germinating coconuts can be eaten fresh.
The shell of the nut can be made into household utensils and decorated pots, converted into shell charcoal (suitable for activation) or used as fuel. Finely ground coconut shell is used as filler for resin glues and moulding powders. Green husks yield white coir (yellow fibres) for making ropes, carpets, mats and geo-textiles. Brown coir from husks of mature fruits is used in brushes (long bristle fibres), mattresses, upholstery and particle board (short fibres). Coir dust or coco peat is a component of potting mixtures (water-holding capacity of 700—900%), light building materials, thermal insulation, adhesives and binders.
A sweet sap containing about 15% sucrose is tapped from unopened inflorescences. It is a refreshing toddy when consumed fresh and it transforms into a light alcoholic wine when fermented. A by-product of palm wine is vinegar. Boiling fresh sap yields palm syrup and sugar. Distillation of palm wine yields a potent arak.
The leaves are used to thatch roofs (Indonesia: 'atap'); the leaflets are plaited into mats, baskets, bags and hats; immature leaflets are made into traditional decorations and small bags or containers for food; the midribs of the leaflets are formed into brooms. The palm heart, which consists of the white, tender tissues of the youngest, unopened leaves at the stem apex, is considered a delicacy. Young palms (3—4 years) have the heaviest palm heart, weighing 6—12 kg.
The wood of old palms is very hard, but a freshly felled trunk can be sawn with a special tungsten carbide-tipped saw blade. Preservative treatment of sawn lumber is needed if it is to be used for construction or any outdoor use. Coconut wood is also suitable for furniture, household utensils and tool handles.
Medicinal uses have been attributed to coconut. The roots are considered anti-pyretic and diuretic. Its decoctions are used against venereal diseases in Malay Peninsula while an infusion is used in Indonesia to treat dysentery. Milk of young coconut is diuretic, laxative, anti-diarrhoeic and counteracts the effects of poison. The oil is used to treat diseased skin and teeth and mixed with other medicines to make embrocations. The kernel of young fruit is mixed with other ingredients and rubbed on the stomach against diarrhoea. The kernel is prepared in Indo-China as a potion to treat ulcers of the skin and the nasal mucous membrane. Coconut palm has also an ornamental value. The palms= often slanting stems and graceful crowns bordering a white beach along a blue sea are hallmarks of the tropics which attract tourists.
Coconut milk or cream (Indonesia/Malaysia: 'santan'; Philippines: 'gata') pressed from freshly grated endosperm mixed with water has been a traditional ingredient in many Asian food and bakery products. It is now also marketed in pasteurized and homogenized canned or powdered form. After preparing coconut milk by boiling grated fresh coconut meat a presscake remains. In Java it is considered a delicacy, named 'blondo' or 'galendo'. Skimmed milk powder, produced after boiling fresh coconut milk and removing the floating oil, contains 25% hydrolyzed starch and can be mixed with water to make a beverage. Protein can be separated by ultrafiltration and spray-dried into a white powder, which is very suitable for infant nutrition. Coconut skim milk is an essential ingredient of a gelatinous delicacy ('nata de coco') in the Philippines used in sweetened desserts, ice-cream and confectionery. Shredded or thinly sliced and desiccated fresh coconut endosperm is a favourite side-dish and ingredient in many confectionery, bakery and snack food products. Ball copra is an Indian speciality produced by slow drying, dehusking and shelling of the whole mature nut. It is used to prepare sweets offered during religious and cultural rites and in traditional medicine.
The nut cavity is filled with water that tastes sweet when the coconut is young. Coconut water is now commercially preserved without altering its typical flavour. It can also be used in the production of 'nata de coco', which is a gelatinous dessert produced by the action of bacteria on coconut water or diluted coconut milk, developed in the Philippines. It is a source of inexpensive growth hormone products for horticulture, such as the Cocogro developed in the Philippines. The tender, jelly-like endosperm of young coconuts is a delicacy consumed directly or grated and mixed with food. The haustorium or apple of germinating coconuts can be eaten fresh.
The shell of the nut can be made into household utensils and decorated pots, converted into shell charcoal (suitable for activation) or used as fuel. Finely ground coconut shell is used as filler for resin glues and moulding powders. Green husks yield white coir (yellow fibres) for making ropes, carpets, mats and geo-textiles. Brown coir from husks of mature fruits is used in brushes (long bristle fibres), mattresses, upholstery and particle board (short fibres). Coir dust or coco peat is a component of potting mixtures (water-holding capacity of 700—900%), light building materials, thermal insulation, adhesives and binders.
A sweet sap containing about 15% sucrose is tapped from unopened inflorescences. It is a refreshing toddy when consumed fresh and it transforms into a light alcoholic wine when fermented. A by-product of palm wine is vinegar. Boiling fresh sap yields palm syrup and sugar. Distillation of palm wine yields a potent arak.
The leaves are used to thatch roofs (Indonesia: 'atap'); the leaflets are plaited into mats, baskets, bags and hats; immature leaflets are made into traditional decorations and small bags or containers for food; the midribs of the leaflets are formed into brooms. The palm heart, which consists of the white, tender tissues of the youngest, unopened leaves at the stem apex, is considered a delicacy. Young palms (3—4 years) have the heaviest palm heart, weighing 6—12 kg.
The wood of old palms is very hard, but a freshly felled trunk can be sawn with a special tungsten carbide-tipped saw blade. Preservative treatment of sawn lumber is needed if it is to be used for construction or any outdoor use. Coconut wood is also suitable for furniture, household utensils and tool handles.
Medicinal uses have been attributed to coconut. The roots are considered anti-pyretic and diuretic. Its decoctions are used against venereal diseases in Malay Peninsula while an infusion is used in Indonesia to treat dysentery. Milk of young coconut is diuretic, laxative, anti-diarrhoeic and counteracts the effects of poison. The oil is used to treat diseased skin and teeth and mixed with other medicines to make embrocations. The kernel of young fruit is mixed with other ingredients and rubbed on the stomach against diarrhoea. The kernel is prepared in Indo-China as a potion to treat ulcers of the skin and the nasal mucous membrane. Coconut palm has also an ornamental value. The palms= often slanting stems and graceful crowns bordering a white beach along a blue sea are hallmarks of the tropics which attract tourists.
Production and International Trade
Average annual world production of copra in 1995—2000 was estimated at about 5.0 million t, equivalent to 3.1 million t oil and 1.7 million t coconut meal. This may represent less than 55% of the actual production from 11 million ha of coconut, owing to considerable home consumption and sales of young coconuts for drinking. Coconut is mainly a smallholder crop and less than 10% of the total area consists of estates. Asia and the Pacific account for 89% of world production, Latin America and the Caribbean for 5% and Africa for 3%. The major coconut producers are the Philippines (39% of world production), Indonesia (24%), India (14%), Mexico (4%), Vietnam and Papua New Guinea (each about 3%). Estimated areas (in million ha) planted with coconut are: Indonesia 3.71, the Philippines 3.16, India 1.67, Sri Lanka 0.49, Thailand 0.41, Malaysia 0.29, Papua New Guinea 0.21 and Vietnam 0.20. With about 1.7 million t of oil traded annually, coconut is now the 7th most important supplier of vegetable oil in the global market. However, it has a special position in the market together with palm-kernel oil as a major source of lauric oil. The Philippines and Papua New Guinea export about 80% of their national coconut oil production in contrast to Indonesia which exports only 20—30% and India which exports almost none. About 50% of the coconut meal produced annually in the world is exported, about 500 000 t by the Philippines and 300 000 t by Indonesia.
Properties
Fresh, mature coconut fruits weigh 1.1—2.5 kg and consist of husk (exocarp and mesocarp) 30—45%, shell (endocarp) 14—16%, endosperm 25—33% and free water in the cavity 13—25%. The proximate composition of fresh endosperm per 100 g edible portion is: water 35—52 g, oil 34—45 g, protein 3—4 g, carbohydrates 9—11 g, fibre 2—3 g and ash 1—2 g. High quality copra has 63—68% oil, no more than 6% water and less than 1% free fatty acid. The fatty acid composition of coconut oil is: caproic acid 0.3—0.5%, caprylic acid 6—8%, capric acid 5—8%, lauric acid 45—50%, myristic acid 15—19%, palmitic acid 8—12%, stearic acid 2—4%, oleic acid 6—8%, linoleic acid 1—2% and arachidic acid 0.5%. More than 90% of the fatty acids are saturated. Lauric acid is an easily digestible source of energy and a precursor of the anti-microbial lipid mono-laurin, which enhances the human immune system. It is hardly deposited at all in body tissues.
Coconut milk contains approximately fat 15—35%, protein 3% and sugar 2%. Powdered coconut milk has: fat 60%, protein 7% and carbohydrates 27%; dried and powdered skim milk has fat 6%, protein 24% and carbohydrates 25%; spray-dried coconut protein powder contains protein 59%. Desiccated coconut contains fat 58%, protein 7% and carbohydrates 24%. Presscake contains fat 6%, protein 21%, carbohydrates 49% and crude fibre 12%. Coconut wood has a density of 400—600 kg/m3, the basal annular outer parts as much as 850 kg/m3; it is suitable as timber for construction purposes because of its moderate to high strength and lack of knots.
Coconut milk contains approximately fat 15—35%, protein 3% and sugar 2%. Powdered coconut milk has: fat 60%, protein 7% and carbohydrates 27%; dried and powdered skim milk has fat 6%, protein 24% and carbohydrates 25%; spray-dried coconut protein powder contains protein 59%. Desiccated coconut contains fat 58%, protein 7% and carbohydrates 24%. Presscake contains fat 6%, protein 21%, carbohydrates 49% and crude fibre 12%. Coconut wood has a density of 400—600 kg/m3, the basal annular outer parts as much as 850 kg/m3; it is suitable as timber for construction purposes because of its moderate to high strength and lack of knots.
Description
An unarmed, unbranched, pleonanthic, monoecious palm tree, with a terminal crown of leaves, up to 20—30 m in tall cultivars, 10—15 m in dwarf cultivars. Roots mostly in the top 1.5 m of soil, normally 6 m x 1 cm but in optimum soil conditions up to 30 m long. Stem cylindrical, erect, often curved or slanting, 20—40 cm in diameter but the swollen base ('bole') up to 60 cm, light grey, becoming bare and conspicuously ringed with scars of fallen leaves. Leaves sheathing, spirally arranged, pinnate, 4.5—6(—7) m long, up to 60—70 per plant of which one half still unfolded in the central spear; petiole stout with clasping, fibrous sheath at base, about one quarter of total leaf length, grooved above, rounded beneath; leaflets 200—250, linear-lanceolate, 50—120 cm x 1.5—5 cm, single folded lengthwise at base, apex acute, regularly arranged in one plane. Inflorescence axillary, protandrous, unopened (immature) looking like a spadix within a spathe, opened (mature) about 1—2 m long, consisting of a central axis with up to 40 lateral, spirally arranged, spike-like rachillae (branches) each bearing 200—300 male flowers and only one to few female flowers near the bare basal part; male flowers 1—3 together, sessile, 0.7—1.3 cm x 0.5—0.7 cm, pale yellow, with 3 small sepals, 3 larger petals, 6 stamens in 2 whorls and a rudimentary pistil; female flowers solitary, much larger than male flowers, globose in bud, ovoid at anthesis, 2—3 cm in diameter, enveloped by 2 small scaly bracteoles, sepals and petals each 3, suborbicular, sub-equal, persistent and enlarging in fruit, pistil with large 3-locular ovary, 3 sessile triangular stigmas and 3 nectaries near ovary base. Fruit a globose, ovoid or ellipsoidal fibrous drupe, indistinctly 3-angled, 20—30 cm long, weighing up to 2.5 kg; exocarp very thin, 0.1 mm thick, smooth, green, brilliant orange, yellow to ivory-coloured when ripe, usually drying to grey-brown in old fruits; mesocarp fibrous, 4—8 cm thick, pale brown; endocarp (shell, together with its contents called the 'nut' of commerce) ovoid, 10—15 cm in diameter, 3—6 mm thick, hard, stony, dark brown, indistinctly 3-angled with 3 longitudinal ridges and 3 large, slightly sunken pores ('eyes') at basal end, each with an operculum. Seed only 1, very large, with a thin brown testa closely appressed to endocarp and adhering firmly to endosperm ('meat') which is firm, 1—2 cm thick, white, oily; at basal end in endosperm a small peglike embryo 0.5—1 cm long is embedded (under one of the endocarp pores); in centre of seed is a large central cavity, partially filled with coconut water which is completely absorbed 6 months after harvesting.
Image
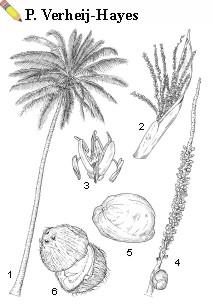 | Cocos nucifera L. - 1, habit fruiting tree [much reduced]; 2, opening inflorescence; 3, male flower; 4, inflorescence branch with male upper part [scars, flower buds] and a female lower part [one flower]; 5, fruit; 6, opened coconut [endocarp] |
Growth and Development
Growth and development Mature fruits of most coconut cultivars start germinating soon after harvest. The embryo enlarges and the apical part emerges from the shell. At the same time, the cotyledon develops into a haustorium. The primary root emerges from the apical mass, followed by the plumule. As growth continues they emerge at opposite sides through the husk. Shoot emergence occurs about 8 weeks after placing coconuts in a germinating bed and another 5 weeks later, the first leaf starts to unfold. The leaves increase in size but remain entire until the seedling has 7—10 leaves, usually after one year=s growth. Subsequent leaves become progressively pinnate.
Tall cultivars produce about 10 leaves during the first year, dwarf palms have 14. In subsequent years, larger and more leaves are formed, until full leaf size is attained and annual production levels off at 12—18 leaves for talls and hybrids and 20—22 leaves for dwarf palms. Since a leaf of a tall palm remains on the tree for about 2.5 years after unfolding, the leaf number in the crown levels off at 30—35 after 6 or 7 years. Leaf initiation until senescence takes about 4 years.
The root system consists of adventitious roots numbering 2000—4000 per palm. Decayed roots are replaced regularly; new roots emerge from the upper part of the thickened basal stem.
The regular development of both canopy and root system is well adapted to the constant environment of the humid lowland tropics. The long development periods of large organs give the palm a certain inflexibility to short-term stress. Under adverse conditions, flowering and fruiting are mainly affected, leading to smaller inflorescences and fewer female flowers; abortion of inflorescences; reduction in fruit set, nut size and filling; premature nut fall and tapering of the stem. Thus, stress affects yield much more than growth. The size of new leaves and roots has been fixed a long time in advance and cannot be adjusted to short-term stress periods. After long-term stress leaf emergence slows down which further reduces yield, since the emergence of inflorescences follows that of the subtending leaves.
At the rosette stage, the growing point continues to enlarge until the size of the leaf initials reflects the prevailing growing conditions; then trunk formation starts. At close spacing, height growth increases at the expense of flowering and fruiting. Precocity and yield are positively correlated with annual leaf formation, as an inflorescence appears in the axil of each leaf. Hence, dwarf varieties yield earlier and more than tall varieties. First flowering in tall varieties occurs at 5—7 years, in dwarf varieties after 2 years and in dwarf x tall hybrids about 3—4 years after germination. Growing conditions have great influence on these aspects. Coconut palms can be more than 100 years old, but highest yields are usually obtained between 10—20 years of age for talls and a few years earlier in dwarfs and hybrids.
During the first phase of anthesis which lasts 16—22 days, only male flowers open progressively from the top to the base of the upper spikes and down to the lowest spikes. Each male flower opens, sheds its pollen and abscises within 2 days. The first female flower at the top of the spadix becomes receptive about 3 weeks in tall or 1 week in dwarf palms after the spathe has opened and the stigmas of the last female flower at the bottom of the spadix turn brown 5—12 days later. Female flowers are nectiferous and sweet-scented. Pollination is both by insects and by wind (the pollen is dry). Each female flower remains receptive for 2—3 days.
Tall coconuts are generally allogamous because the male and female phases do not overlap while in dwarfs, self-pollination is common due to considerable overlap. Self-pollination can also occur when the female phase of one inflorescence overlaps with the male phase of a second inflorescence on the same tree. About 50—70% of the female flowers abort during the first two months due to poor fertilization or other physiological causes. Fruits are mature 11—12 months after anthesis, but may not drop until 15 months old.
Tall cultivars produce about 10 leaves during the first year, dwarf palms have 14. In subsequent years, larger and more leaves are formed, until full leaf size is attained and annual production levels off at 12—18 leaves for talls and hybrids and 20—22 leaves for dwarf palms. Since a leaf of a tall palm remains on the tree for about 2.5 years after unfolding, the leaf number in the crown levels off at 30—35 after 6 or 7 years. Leaf initiation until senescence takes about 4 years.
The root system consists of adventitious roots numbering 2000—4000 per palm. Decayed roots are replaced regularly; new roots emerge from the upper part of the thickened basal stem.
The regular development of both canopy and root system is well adapted to the constant environment of the humid lowland tropics. The long development periods of large organs give the palm a certain inflexibility to short-term stress. Under adverse conditions, flowering and fruiting are mainly affected, leading to smaller inflorescences and fewer female flowers; abortion of inflorescences; reduction in fruit set, nut size and filling; premature nut fall and tapering of the stem. Thus, stress affects yield much more than growth. The size of new leaves and roots has been fixed a long time in advance and cannot be adjusted to short-term stress periods. After long-term stress leaf emergence slows down which further reduces yield, since the emergence of inflorescences follows that of the subtending leaves.
At the rosette stage, the growing point continues to enlarge until the size of the leaf initials reflects the prevailing growing conditions; then trunk formation starts. At close spacing, height growth increases at the expense of flowering and fruiting. Precocity and yield are positively correlated with annual leaf formation, as an inflorescence appears in the axil of each leaf. Hence, dwarf varieties yield earlier and more than tall varieties. First flowering in tall varieties occurs at 5—7 years, in dwarf varieties after 2 years and in dwarf x tall hybrids about 3—4 years after germination. Growing conditions have great influence on these aspects. Coconut palms can be more than 100 years old, but highest yields are usually obtained between 10—20 years of age for talls and a few years earlier in dwarfs and hybrids.
During the first phase of anthesis which lasts 16—22 days, only male flowers open progressively from the top to the base of the upper spikes and down to the lowest spikes. Each male flower opens, sheds its pollen and abscises within 2 days. The first female flower at the top of the spadix becomes receptive about 3 weeks in tall or 1 week in dwarf palms after the spathe has opened and the stigmas of the last female flower at the bottom of the spadix turn brown 5—12 days later. Female flowers are nectiferous and sweet-scented. Pollination is both by insects and by wind (the pollen is dry). Each female flower remains receptive for 2—3 days.
Tall coconuts are generally allogamous because the male and female phases do not overlap while in dwarfs, self-pollination is common due to considerable overlap. Self-pollination can also occur when the female phase of one inflorescence overlaps with the male phase of a second inflorescence on the same tree. About 50—70% of the female flowers abort during the first two months due to poor fertilization or other physiological causes. Fruits are mature 11—12 months after anthesis, but may not drop until 15 months old.
Other Botanical Information
Cocos nucifera is the only species of the genus Cocos L. A generally accepted classification system for the wide variability of coconut does not exist. Coconuts that are thought to be of natural origin are said to be of the 'Niu kafa type' (fruits long, angular, thick husked, floating easily, long lasting viability, slow germination); those which are thought to be developed under cultivation are of the 'Niu vai type' (fruits globose, thinner husk, not floating easily, increased endosperm, earlier germination). Niu kafa and niu vai are Polynesian words. Where these 2 types come into contact, introgression takes place.
Up to now, cultivated coconuts have been classified into 2 groups: tall palms (sometimes referred to as var. typica Nar.) and dwarf palms (sometimes referred to as var. nana (Griff.) Nar.). More than 95% of all cultivated coconuts are tall palms. Examples of tall cultivars are: 'Malayan Tall', 'Rennell Island Tall', 'Vanuatu Tall', 'Jamaican Tall' and 'West African Tall'. Dwarf palms are rare, but can be found in different ecotypes. Characteristics of dwarf palms are: weaker growth and slow height increment; slender stem; smaller leaves, inflorescences and fruits; precocity and rapid succession of inflorescences; high degree of self-pollination. The inheritance of dwarfness is not well understood but hybrids are usually intermediate in height increment and other characteristics to the tall and dwarf parents. Three different types of dwarf cultivars exist: the 'Niu Leka' from Fiji which differs only from the talls by its very short internodes and short rigid leaves, while it is also allogamous; the medium-sized palms such as 'Malayan Dwarf' from Indonesia, 'Gangabondam' from India and 'King' from Sri Lanka; and the small dwarf cultivars in various countries. Dwarfs are also differentiated based on the colour of leaf petiole of young coconuts, into green, yellow and red (orange or golden) dwarfs. In the Philippines, there are green dwarfs with large fruits, such as 'Tacunan', 'Kinabalan' and 'Catigan'.
The nuts of 'Makapuno' from the Philippines and the 'Kelapa Kopjor' from Indonesia have endosperms that fill almost the entire cavity. The endosperm is soft, has a peculiar taste and is considered a delicacy. The nuts do not germinate but the embryos can be cultured in vitro. This character may appear in any tall cultivar.
Classification in cultivated coconuts can best be done by distinguishing cultivar groups and cultivars. A promising classification system of coconut cultivars is based on the degree of introgression, which can be expressed in characteristics of the fruits: proportion of husk in the whole fruit, proportions of water, meat and shell in the husked fruit.
Up to now, cultivated coconuts have been classified into 2 groups: tall palms (sometimes referred to as var. typica Nar.) and dwarf palms (sometimes referred to as var. nana (Griff.) Nar.). More than 95% of all cultivated coconuts are tall palms. Examples of tall cultivars are: 'Malayan Tall', 'Rennell Island Tall', 'Vanuatu Tall', 'Jamaican Tall' and 'West African Tall'. Dwarf palms are rare, but can be found in different ecotypes. Characteristics of dwarf palms are: weaker growth and slow height increment; slender stem; smaller leaves, inflorescences and fruits; precocity and rapid succession of inflorescences; high degree of self-pollination. The inheritance of dwarfness is not well understood but hybrids are usually intermediate in height increment and other characteristics to the tall and dwarf parents. Three different types of dwarf cultivars exist: the 'Niu Leka' from Fiji which differs only from the talls by its very short internodes and short rigid leaves, while it is also allogamous; the medium-sized palms such as 'Malayan Dwarf' from Indonesia, 'Gangabondam' from India and 'King' from Sri Lanka; and the small dwarf cultivars in various countries. Dwarfs are also differentiated based on the colour of leaf petiole of young coconuts, into green, yellow and red (orange or golden) dwarfs. In the Philippines, there are green dwarfs with large fruits, such as 'Tacunan', 'Kinabalan' and 'Catigan'.
The nuts of 'Makapuno' from the Philippines and the 'Kelapa Kopjor' from Indonesia have endosperms that fill almost the entire cavity. The endosperm is soft, has a peculiar taste and is considered a delicacy. The nuts do not germinate but the embryos can be cultured in vitro. This character may appear in any tall cultivar.
Classification in cultivated coconuts can best be done by distinguishing cultivar groups and cultivars. A promising classification system of coconut cultivars is based on the degree of introgression, which can be expressed in characteristics of the fruits: proportion of husk in the whole fruit, proportions of water, meat and shell in the husked fruit.
Ecology
Coconut palm is essentially a crop of the humid tropics. It is fairly adaptable with regard to temperature and water supply and so highly valued that it is still common near the limits of its ecological zone. The annual sunlight requirement is above 2000 hours, with a likely lower limit of 120 hours per month. The optimum mean annual temperature is estimated at 27°C with average diurnal variation of 5—7°C. For good yields, a minimum monthly mean temperature of 20°C is required. Temperatures below 7°C may seriously damage young palms, but cultivars differ in their tolerance of low temperature. While most coconuts are planted in areas below 500 m, palms may thrive at altitudes up to 1000 m, although low temperatures will affect growth and yield.
Generally, palms grow in areas with evenly distributed annual rainfall of 1000—2000 mm and high relative humidity, but they can still survive in drier regions if there is adequate soil moisture. The semi-xerophytic leaves enable the coconut palm to minimize water loss and withstand drought for several months. In India, a monthly rainfall of 150 mm (with only a 3-month dry period) is enough, while in the Philippines, rainfall of 125—195 mm (1500—2300 mm annually) is ideal.
The coconut palm thrives in a wide range of soils, from coarse sand to clay, if soils have adequate drainage and aeration. Coconut palms are halophytic and tolerate salt in the soil well. Coconut can grow in soils with a wide range of pH but grows best at pH 5.5—7.
Generally, palms grow in areas with evenly distributed annual rainfall of 1000—2000 mm and high relative humidity, but they can still survive in drier regions if there is adequate soil moisture. The semi-xerophytic leaves enable the coconut palm to minimize water loss and withstand drought for several months. In India, a monthly rainfall of 150 mm (with only a 3-month dry period) is enough, while in the Philippines, rainfall of 125—195 mm (1500—2300 mm annually) is ideal.
The coconut palm thrives in a wide range of soils, from coarse sand to clay, if soils have adequate drainage and aeration. Coconut palms are halophytic and tolerate salt in the soil well. Coconut can grow in soils with a wide range of pH but grows best at pH 5.5—7.
Propagation and planting
Coconut palm is propagated by seed which is recalcitrant. The multiplication factor is low, as one tall palm will in general not produce more than 100—200 seed-nuts per year. Although coconut plants can be regenerated through somatic embryogenesis, genotypic differences in rate of embryo formation and difficulties in hardening of in-vitro plants have been a constraints to practical methods of large-scale clonal propagation so far. However, a field of clonal coconuts may soon be planted in Mexico. In vitro culture of excised embryos is also possible. It solves problems of plant quarantine restrictions and finds application in the international exchange of germplasm.
Seed-nuts are usually given a resting period of one month after harvesting. They are kept in a germination bed from where uniform seedlings can be transplanted to polythene bags or to nursery beds. The polybag method and regular fertilization have largely replaced the bare-root seedlings raised in beds. Seedlings that are 3—8 months old are transplanted in the field. They can be kept longer in the nursery bed but will then sustain a greater transplanting shock. Coconut is planted mostly at spacings of 8—10 m x 8—10 m, in a triangular or square system. Dwarf cultivars are planted at a spacing of 7.5 m x 7.5 m. Hedge planting may be used to facilitate intercropping, but the radial symmetry of the leaf arrangement does not tolerate extreme forms of row cropping.
Growers prefer wider palm spacing to prevent inter-tree competition. As the open crowns also transmit a fair portion of incident light coconut is well suited to intercropping. Coconut is occasionally grown with cocoa and coffee. Although this usually results in lower copra yields, the combined income from well-fertilized coconut and intercrop is much higher than that from coconut alone. In humid climates, cocoa is one of the best intercrops. In Malaysia, more than 1000 kg/ha of dry beans have been obtained from cocoa grown under coconuts. Coconut is also grown in mixed cropping systems with other trees like rubber, mango, cashew and banana. Under coconuts in the Philippines yields of bananas of 40—60 t/ha have been obtained. Pastures are sometimes established under the palms for use in mixed husbandry and green manures are occasionally planted. However, pasture and cover crops can only be grown and maintained when there is sufficient rain. Catch crops such as rice, maize, finger millet, sweet potato, cassava, vegetables and spices are often planted until the palms come into bearing. These crops should not be planted closer than 2 m to the palms.
Seed-nuts are usually given a resting period of one month after harvesting. They are kept in a germination bed from where uniform seedlings can be transplanted to polythene bags or to nursery beds. The polybag method and regular fertilization have largely replaced the bare-root seedlings raised in beds. Seedlings that are 3—8 months old are transplanted in the field. They can be kept longer in the nursery bed but will then sustain a greater transplanting shock. Coconut is planted mostly at spacings of 8—10 m x 8—10 m, in a triangular or square system. Dwarf cultivars are planted at a spacing of 7.5 m x 7.5 m. Hedge planting may be used to facilitate intercropping, but the radial symmetry of the leaf arrangement does not tolerate extreme forms of row cropping.
Growers prefer wider palm spacing to prevent inter-tree competition. As the open crowns also transmit a fair portion of incident light coconut is well suited to intercropping. Coconut is occasionally grown with cocoa and coffee. Although this usually results in lower copra yields, the combined income from well-fertilized coconut and intercrop is much higher than that from coconut alone. In humid climates, cocoa is one of the best intercrops. In Malaysia, more than 1000 kg/ha of dry beans have been obtained from cocoa grown under coconuts. Coconut is also grown in mixed cropping systems with other trees like rubber, mango, cashew and banana. Under coconuts in the Philippines yields of bananas of 40—60 t/ha have been obtained. Pastures are sometimes established under the palms for use in mixed husbandry and green manures are occasionally planted. However, pasture and cover crops can only be grown and maintained when there is sufficient rain. Catch crops such as rice, maize, finger millet, sweet potato, cassava, vegetables and spices are often planted until the palms come into bearing. These crops should not be planted closer than 2 m to the palms.
Husbandry
Weeding is essential, especially for young coconut palms. Green manuring is often practised to advantage. Fertilizing is required, especially on soils that have been cultivated for many years, but smallholders seldom apply fertilizers. If nutrient deficiencies largely limit growth and yield, responses to fertilizer application and other cultural practices can be observed within one year. Potassium and chloride are the major nutrients needed by the palm, followed by nitrogen, phosphorous and sulphur. Leaf analysis is an acceptable and quick guide to the fertilizer requirements of the palm. The annual crop nutrient removal of one hectare of coconuts, yielding 7000 nuts, is about: 49 kg N, 16 kg P2O5, 115 kg K2O, 5 kg Ca, 8 kg Mg, 11 kg Na, 64 kg Cl and 4 kg S. An example of a yearly fertilizer recommendation per palm is a mixture of 0.4 kg N, 0.3 kg P2O5, 1.2 kg K2O, 0.20 kg S and 0.90 kg Cl, applied in split applications in a band around the palm (l.0—1.5 m from the trunk) and split into 2 applications, at the beginning and end of the rainy season. Fertilizer doses depend on local conditions. Foliar and soil analyses help to determine the nutrient status of the palms. In several countries in Asia sea salt (NaCl) is commonly applied to coconut palms with positive effects on yields.
Irrigation is sometimes practised in dry areas where water is available and sea water may be applied occasionally as long as the salt content in the soil does not rise too high.
Irrigation is sometimes practised in dry areas where water is available and sea water may be applied occasionally as long as the salt content in the soil does not rise too high.
Diseases and Pests
Many diseases affect coconut palm. Important are yellowing diseases, such as lethal yellowing in the Caribbean, Cape St. Paul wilt, Kaincopé disease, Kribi disease in West Africa and lethal disease in Tanzania. These are caused by mycoplasma-like organisms. Generally, the symptoms of yellowing diseases are browning and collapse of spear leaves, yellowing of mature leaves, collapse of roots, premature nut fall, death of bud and later, of the tree. 'Malayan Dwarf' is highly tolerant of lethal yellowing but shows varying tolerance of other yellowing diseases. Tall palms are more susceptible.
Similar diseases of unknown etiology but suspected to be caused by mycoplasma-like organisms are Malaysian wilt in Malaysia, stem necrosis in Malaysia and Indonesia, Natuna wilt and leaf yellowing disease in Indonesia, Socorro wilt in the Philippines and the New Hebrides disease in Vanuatu. Malaysian wilt is characterized by premature nut fall; stiff, yellowish and smaller new leaves; wilting and drying of old leaves and eventual death of the palm. Palms with stem necrosis show shorter young leaves, die-back of leaflets, necrosis in the leaflet midrib, internal disorganization and necrosis of stem, bud, inflorescence and roots. Natuna wilt causes leaf wilting and bending in both young and old trees until leaves fall simultaneously with the nuts. Leaf yellowing disease causes intensive yellowing of the older leaves and at the advanced stage, the leaves are smaller and the whole crown is stunted. Symptoms of Socorro wilt are premature senescence of the outer whorl of hanging leaves or the drying of the leaves from the tip to the base until they drop, premature falling of nuts, failure of inflorescence to develop, and spathes becoming brown and dying. The few nuts that develop are usually deformed, oblong, small and with damaged kernels.
Kerala wilt, possibly caused by a virus, is an important disease in India. Cadang-cadang, caused by the cadang-cadang viroid (CCVD) is a devastating disease especially of flowering palms in the Philippines, particularly in the Bicol region and adjacent provinces (estimates of the affected area range from 250 000 ha to 400 000 ha). The symptoms are yellow mottling on leaves, formation of small and stiff leaves that usually break at the middle until only a group of small, erect and yellowish-green leaves remain at the top of the stem and production of fewer roots. The inflorescence is also affected; at the later stage of the disease, only male flowers develop and nut production stops. Although control methods are still unknown, the eradication of diseased palms and sterilization of knives that are used on the farms may help reduce the spread and incidence of cadang-cadang. Coconuts in Guam are infected by a disease similar to cadang-cadang, also caused by a viroid.
Bud rot occurs worldwide and is caused by the soil-borne fungus Phytophthora palmivora which is favoured by high humidity. It causes rotting of the spear and the growing point. It can be controlled by wider plant spacing, better aeration, drainage and weed control. Basal stem rot develops from an infection by the fungus Ganoderma boninense. The fungus first affects and destroys the roots and then the base of the stem turns reddish-brown and releases a brown, gummy exudate. Disease occurrence can be prevented through improved growing conditions, production techniques and proper sanitation measures. Control methods are eradication of affected palms and application of fungicide. Stem bleeding or oozing of reddish-brown liquid from the cracked stem is caused by Thielaviopsis paradoxa. Cultural management techniques and drenching the soil with fungicides effectively control the disease. Leaf blight caused by Pestalotia palmarum and leaf rot or leaf spot caused by Drechslera halodes (= Drechslera incurvata) are widespread fungal diseases, while leaf blight caused by Botryodiplodia theobromae damages palms in Brazil, Malaysia, Sri Lanka and Trinidad especially during months of high temperatures and low relative humidity and rainfall.
Numerous insect pests attack coconut palms. The rhinoceros beetle (Oryctes rhinoceros) is widespread in South-East Asia and the Pacific. Its larvae tunnel through the apical bud leaving characteristic triangular cuts in opened leaves. When the growing point is attacked, the palm dies. Control is done by keeping the plantation clean and applying Baculovirus oryctes or the insect-pathogenic fungus Metarhizium anisopliaeto breeding places. Recently, a male-produced aggregation pheromone was discovered to be a powerful attractant in selective trapping to reduce beetle populations. Different outbreak situations require specific control approaches. Other Coleoptera that inflict serious damage to coconut are Promecotheca spp. in Indonesia, Malaysia and the Philippines and Brontispa longissima in Indonesia, Malaysia and the Pacific. Larvae of Promecothecaspp. tunnel through foliar tissues while adults eat the underside of leaves. Brontispa longissima larvae and adults feed on leaflet tissues and may defoliate the whole crown in severe cases. The weevils Rhynchophorus ferrugineus and Rhynchophorus schach in South Asia and Malaysia cause serious damage by their boring into the coconut stem. Many caterpillars feed on coconut leaves, such as Hidari irava in Indonesia and Malaysia, Tirathaba spp. in South-East Asia and the Pacific, Setoria nitens in Burma (Myanmar), Indonesia, Malaysia and Vietnam, Parasa lepida in India, New Guinea, China and South-East Asia and Brachartona (Artona) catoxantha in Indonesia, Malaysia, New Guinea and the Philippines. The scale insect Aspidiotus destructor is one of the most widespread pests of the coconut palm and can be controlled by an emulsion of soft laundry soap and kerosene in water. The white fly Aleurodicus destructor sometimes causes serious damage to coconut in Indonesia and the Philippines.
Similar diseases of unknown etiology but suspected to be caused by mycoplasma-like organisms are Malaysian wilt in Malaysia, stem necrosis in Malaysia and Indonesia, Natuna wilt and leaf yellowing disease in Indonesia, Socorro wilt in the Philippines and the New Hebrides disease in Vanuatu. Malaysian wilt is characterized by premature nut fall; stiff, yellowish and smaller new leaves; wilting and drying of old leaves and eventual death of the palm. Palms with stem necrosis show shorter young leaves, die-back of leaflets, necrosis in the leaflet midrib, internal disorganization and necrosis of stem, bud, inflorescence and roots. Natuna wilt causes leaf wilting and bending in both young and old trees until leaves fall simultaneously with the nuts. Leaf yellowing disease causes intensive yellowing of the older leaves and at the advanced stage, the leaves are smaller and the whole crown is stunted. Symptoms of Socorro wilt are premature senescence of the outer whorl of hanging leaves or the drying of the leaves from the tip to the base until they drop, premature falling of nuts, failure of inflorescence to develop, and spathes becoming brown and dying. The few nuts that develop are usually deformed, oblong, small and with damaged kernels.
Kerala wilt, possibly caused by a virus, is an important disease in India. Cadang-cadang, caused by the cadang-cadang viroid (CCVD) is a devastating disease especially of flowering palms in the Philippines, particularly in the Bicol region and adjacent provinces (estimates of the affected area range from 250 000 ha to 400 000 ha). The symptoms are yellow mottling on leaves, formation of small and stiff leaves that usually break at the middle until only a group of small, erect and yellowish-green leaves remain at the top of the stem and production of fewer roots. The inflorescence is also affected; at the later stage of the disease, only male flowers develop and nut production stops. Although control methods are still unknown, the eradication of diseased palms and sterilization of knives that are used on the farms may help reduce the spread and incidence of cadang-cadang. Coconuts in Guam are infected by a disease similar to cadang-cadang, also caused by a viroid.
Bud rot occurs worldwide and is caused by the soil-borne fungus Phytophthora palmivora which is favoured by high humidity. It causes rotting of the spear and the growing point. It can be controlled by wider plant spacing, better aeration, drainage and weed control. Basal stem rot develops from an infection by the fungus Ganoderma boninense. The fungus first affects and destroys the roots and then the base of the stem turns reddish-brown and releases a brown, gummy exudate. Disease occurrence can be prevented through improved growing conditions, production techniques and proper sanitation measures. Control methods are eradication of affected palms and application of fungicide. Stem bleeding or oozing of reddish-brown liquid from the cracked stem is caused by Thielaviopsis paradoxa. Cultural management techniques and drenching the soil with fungicides effectively control the disease. Leaf blight caused by Pestalotia palmarum and leaf rot or leaf spot caused by Drechslera halodes (= Drechslera incurvata) are widespread fungal diseases, while leaf blight caused by Botryodiplodia theobromae damages palms in Brazil, Malaysia, Sri Lanka and Trinidad especially during months of high temperatures and low relative humidity and rainfall.
Numerous insect pests attack coconut palms. The rhinoceros beetle (Oryctes rhinoceros) is widespread in South-East Asia and the Pacific. Its larvae tunnel through the apical bud leaving characteristic triangular cuts in opened leaves. When the growing point is attacked, the palm dies. Control is done by keeping the plantation clean and applying Baculovirus oryctes or the insect-pathogenic fungus Metarhizium anisopliaeto breeding places. Recently, a male-produced aggregation pheromone was discovered to be a powerful attractant in selective trapping to reduce beetle populations. Different outbreak situations require specific control approaches. Other Coleoptera that inflict serious damage to coconut are Promecotheca spp. in Indonesia, Malaysia and the Philippines and Brontispa longissima in Indonesia, Malaysia and the Pacific. Larvae of Promecothecaspp. tunnel through foliar tissues while adults eat the underside of leaves. Brontispa longissima larvae and adults feed on leaflet tissues and may defoliate the whole crown in severe cases. The weevils Rhynchophorus ferrugineus and Rhynchophorus schach in South Asia and Malaysia cause serious damage by their boring into the coconut stem. Many caterpillars feed on coconut leaves, such as Hidari irava in Indonesia and Malaysia, Tirathaba spp. in South-East Asia and the Pacific, Setoria nitens in Burma (Myanmar), Indonesia, Malaysia and Vietnam, Parasa lepida in India, New Guinea, China and South-East Asia and Brachartona (Artona) catoxantha in Indonesia, Malaysia, New Guinea and the Philippines. The scale insect Aspidiotus destructor is one of the most widespread pests of the coconut palm and can be controlled by an emulsion of soft laundry soap and kerosene in water. The white fly Aleurodicus destructor sometimes causes serious damage to coconut in Indonesia and the Philippines.
Harvesting
Coconut fruits can be harvested about 11—12 months after flowering. The palm can be harvested every 2—3 months but rapidly germinating types should be harvested more frequently. Dwarf cultivars sprout in 45—60 days and must be harvested monthly. Climbing the palms and cutting the ripe bunches is still the harvesting method most practised. Gathering fallen nuts is easier, but there are more losses due to rat attack and theft. Some nuts may germinate on the tree and consequently, their kernel and oil content may have started to deteriorate. In some countries bamboo poles (up to 25 m long) with a knife attached to the top end are used to cut the ripe bunches, elsewhere monkeys (Macacus nemestrina) are trained to harvest ripe nuts.
Yield
Smallholder plantations usually yield between 0.5—1 t of copra/ha. In Malaysia, average estate yields are about 1.5 t of copra/ha but the potential yield is about 3.5 t/ha. Well-managed plantations of selected local tall coconut palms in Indonesia yield 3.5—4.5 t copra /ha. Rehabilitated and fertilized tall coconut palms in small farms in the Philippines achieve an average annual yield of 2.8 t copra/ha or 83 nuts/tree per year. Plantations of dwarf coconut palms in Malaysia produce about 1.5—2 t/ha and even 3.5 t/ha under favourable conditions. Dwarf x tall hybrids combine the high number of fruits produced by the dwarf type with the larger size from the tall one and usually have a higher yielding potential than the parents. Experimental yields of more than 6—9 t/ha have been obtained in Ivory Coast and the Philippines.
Handling After Harvest
Harvested coconuts are stored in a protected place until the husks are completely dry. Dried coconuts are dehusked manually by striking and twisting them on a steel point that is placed firmly in the ground. Dehusking machines have been developed but have not been a success. After dehusking, nuts are split with a machete and the water is drained. The nut halves are placed in a kiln dryer or an indirect hot air dryer for 1—2 days, after which the endosperm is scooped out from the shell and dried further until its moisture content is less than 6%. Sun-drying is also practised but there is a higher risk of product deterioration especially during humid and rainy periods. Aflatoxin-producing moulds may affect the quality when the moisture content of dried copra exceeds 12%.
Coconut oil can be extracted from the copra (yield about 60%) by dry processing methods such as mechanical pressing and by using solvents. It can also be extracted from the fresh kernel through several wet processes. In traditional extraction coconut cream or milk is obtained from the grated fresh kernel by boiling it gently until the oil floats to the surface.
Whole or dehusked coconuts are also sold to coconut desiccation factories. To produce desiccated coconut, the shell and the brown testa are pared off, the white endosperm washed, steamed, pasteurized, shredded into small pieces of various sizes and forms, dried and packed.
Coconut oil can be extracted from the copra (yield about 60%) by dry processing methods such as mechanical pressing and by using solvents. It can also be extracted from the fresh kernel through several wet processes. In traditional extraction coconut cream or milk is obtained from the grated fresh kernel by boiling it gently until the oil floats to the surface.
Whole or dehusked coconuts are also sold to coconut desiccation factories. To produce desiccated coconut, the shell and the brown testa are pared off, the white endosperm washed, steamed, pasteurized, shredded into small pieces of various sizes and forms, dried and packed.
Genetic Resources
Local coconut cultivars (ecotypes) are usually heterogeneous populations with some predominating characteristics. Cultivars with different names and growing in different areas are sometimes rather similar and maybe of the same origin. Germplasm collections are found in several research stations around the world. In 1978, the International Board for Plant Genetic Resources (IBPGR, now IPGRI) adopted a minimum list of descriptors to be used in collecting germplasm in the field. In 1980, it supported the survey and collection of coconut germplasm in priority areas in South-East Asia and provided funds for the collection of coconuts in Indonesia, the establishment of a coconut germplasm centre in the Philippines and collection of germplasm in the Pacific to be planted on one of the Andaman Islands to screen for Kerala wilt disease resistance for mainland India.
The Coconut Genetic Resources Network (COGENT), with IPGRI=s administrative support, coordinates the conservation of more than 700 accessions in 15 countries. Major coconut germplasm collections include those of the Philipine Coconut Authority (PCA), the Research and Development Centre for Industrial Crops (RDCIC) in Indonesia, IPGRI-Asia, the Pacific and Oceania at Serdang, Malaysia, the Central Plantation Crop Institute (CPCRI) in India, the National Centre for Agricultural Research (CNRA) in Ivory Coast and the National Coconut Development Programme (NCDP) in Tanzania.
Germplasm conservation by field collections requires considerable resources of land, staff and upkeep and remains vulnerable to natural disasters and diseases. The cryopreservation of coconut embryos and pollen will enable the safe and inexpensive long-term storage of coconut genetic resources.
The Coconut Genetic Resources Network (COGENT), with IPGRI=s administrative support, coordinates the conservation of more than 700 accessions in 15 countries. Major coconut germplasm collections include those of the Philipine Coconut Authority (PCA), the Research and Development Centre for Industrial Crops (RDCIC) in Indonesia, IPGRI-Asia, the Pacific and Oceania at Serdang, Malaysia, the Central Plantation Crop Institute (CPCRI) in India, the National Centre for Agricultural Research (CNRA) in Ivory Coast and the National Coconut Development Programme (NCDP) in Tanzania.
Germplasm conservation by field collections requires considerable resources of land, staff and upkeep and remains vulnerable to natural disasters and diseases. The cryopreservation of coconut embryos and pollen will enable the safe and inexpensive long-term storage of coconut genetic resources.
Breeding
Breeding methods common to cross-pollinating species are applied to coconut palm. The long duration of one breeding generation (more than 10 years), low multiplication rate (1 : 50/100), recalcitrant and large 'seed' and the large areas of land required for field testing, are major obstacles to rapid selection progress. About 95% of all planted coconut palms in the world are open-pollinated progenies after mass selection within local ecotypes, often informally applied by the growers themselves.
Important selection criteria in coconut are: yield (kg copra per ha) and its components (number of nuts, copra content per nut), early production, disease resistance and drought tolerance. Selection for endosperm thickness is a minor factor of selection; oil content and quality are fairly constant; length of husk fibres is a selection criterion in Sri Lanka only. The flavour of immature coconut water varies with ecotypes, but has not been a criterion for formal selection as yet.
The genetic variance in coconut yield and its components is mainly due to additive genetic effects and the superior hybrids are the result of the general combining ability of the parents. Methods of (reciprocal) recurrent selection with genetically diverse subpopulations (dwarfs and talls) are now used in some coconut breeding programmes to increase substantial transgressive hybrid vigour for yield in new cultivars. Chemical and more recently, molecular markers are applied in coconut breeding to measure genetic divergence between sub-populations.
Dwarf x tall hybrids have considerable heterosis for yield and precocity, hence the focus of breeding programmes of several coconut research centres on such hybrids since 1960. Some 400 hybrids have been tested worldwide during the last 35 years; about 10 of these internationally at several locations. The coconut research centre at Port Bouet in Ivory Coast tested 123 hybrids, of which 35 produced 65% more than the 'West African Tall' standard cultivar. Four hybrids yielded even more than twice as much (3.4—4.5 t/ha copra), including PB121 ('Malayan Yellow Dwarf' x 'West African Tall') which has been planted widely also in South-East Asia. Host resistance to major diseases has high priority in some areas, but sources of resistance are not always available in the coconut, e.g. against Cadang-cadang disease in the Philippines.
Crosses for breeding purposes are made by hand pollination after emasculation and bagging of inflorescences. Pollen collected from the male parent can be stored (dry and under vacuum) for a considerable period. Large-scale seed production is based on pollination of previously emasculated inflorescences (not bagged) in isolated seed gardens planted solely with the female parent of the hybrid cultivar (usually a dwarf type). One hectare of seed garden produces yearly enough 'seed' for planting 50—60 ha only. Hybrid seed production is rather expensive and requires large land areas. An estimated 15% of all coconut palms planted during the last decade are hybrids. Examples of widely planted hybrid cultivars are: KB and KHINA series in Indonesia; the PCA 15 series in the Philippines; Sawi-1 (= PB121) and Chumphon 60 in Thailand and PB series (e.g. PB121) from Ivory Coast.
In some areas of the Philippines, the more robust tall palms are preferred as planting material. In 1992, a programme of 15 crosses between 6 tall types was carried out and best selected F1 was planted in a seed garden to produce open-pollinated F2 seeds with similar qualities as a synthetic (hybrid) cultivar. Cost of seed production is much lower, as no emasculation or artificial pollination are involved.
Important selection criteria in coconut are: yield (kg copra per ha) and its components (number of nuts, copra content per nut), early production, disease resistance and drought tolerance. Selection for endosperm thickness is a minor factor of selection; oil content and quality are fairly constant; length of husk fibres is a selection criterion in Sri Lanka only. The flavour of immature coconut water varies with ecotypes, but has not been a criterion for formal selection as yet.
The genetic variance in coconut yield and its components is mainly due to additive genetic effects and the superior hybrids are the result of the general combining ability of the parents. Methods of (reciprocal) recurrent selection with genetically diverse subpopulations (dwarfs and talls) are now used in some coconut breeding programmes to increase substantial transgressive hybrid vigour for yield in new cultivars. Chemical and more recently, molecular markers are applied in coconut breeding to measure genetic divergence between sub-populations.
Dwarf x tall hybrids have considerable heterosis for yield and precocity, hence the focus of breeding programmes of several coconut research centres on such hybrids since 1960. Some 400 hybrids have been tested worldwide during the last 35 years; about 10 of these internationally at several locations. The coconut research centre at Port Bouet in Ivory Coast tested 123 hybrids, of which 35 produced 65% more than the 'West African Tall' standard cultivar. Four hybrids yielded even more than twice as much (3.4—4.5 t/ha copra), including PB121 ('Malayan Yellow Dwarf' x 'West African Tall') which has been planted widely also in South-East Asia. Host resistance to major diseases has high priority in some areas, but sources of resistance are not always available in the coconut, e.g. against Cadang-cadang disease in the Philippines.
Crosses for breeding purposes are made by hand pollination after emasculation and bagging of inflorescences. Pollen collected from the male parent can be stored (dry and under vacuum) for a considerable period. Large-scale seed production is based on pollination of previously emasculated inflorescences (not bagged) in isolated seed gardens planted solely with the female parent of the hybrid cultivar (usually a dwarf type). One hectare of seed garden produces yearly enough 'seed' for planting 50—60 ha only. Hybrid seed production is rather expensive and requires large land areas. An estimated 15% of all coconut palms planted during the last decade are hybrids. Examples of widely planted hybrid cultivars are: KB and KHINA series in Indonesia; the PCA 15 series in the Philippines; Sawi-1 (= PB121) and Chumphon 60 in Thailand and PB series (e.g. PB121) from Ivory Coast.
In some areas of the Philippines, the more robust tall palms are preferred as planting material. In 1992, a programme of 15 crosses between 6 tall types was carried out and best selected F1 was planted in a seed garden to produce open-pollinated F2 seeds with similar qualities as a synthetic (hybrid) cultivar. Cost of seed production is much lower, as no emasculation or artificial pollination are involved.
Prospects
Some of the latest dwarf x tall hybrid cultivars of coconut palms can potentially yield more than 6 t/ha of copra per year (3.7 t of oil), but coconut palm does not appear to have a bright future as a plantation crop in the long term. Coconut oil already faces increasing competition in the world market from palm-kernel oil and both may eventually also be partly replaced by lauric oils produced by genetically transformed soya bean and brassica oilseed. On the other hand, as a smallholder crop in the coastal areas of the tropics, coconut will continue to be a very important supplier of multifunctional food and other products. Sometimes, it is practically the only crop that can be grown in the prevailing ecosystem (e.g. some Pacific Islands). A quickly growing world market for healthy and environmentally friendly products should offer new opportunities for the coconut export trade. However, this will require astute marketing, more research into the economic viability of smallholder coconut production systems (e.g. replanting, mixed intercropping and biological control of diseases and pests) and into novel processing technologies for local industries to manufacture diversified coconut products suitable for the international market.
Literature
Batugal, P.A. & Rao, V.R. (Editors), 1994. Coconut breeding. Workshop on Standardization of Coconut Breeding Research Techniques, 20—25 June 1994, Port Bouet, Côte d'Ivoire. International Plant Genetics Resources Institute, Regional Office for Asia, the Pacific and Oceania, Serdang, Malaysia 150 pp.
Bourdeix, R., Baudouin, L., Billotte, N., Labouise, J.P. & Noiret, J.M., 1997. Le Cocotier [The Coconut]. In: Charrier, A., Jackot, M., Hamon, S. & Nicolas, D. (Editors): L'Amélioration des plants tropicales [Tropical plant breeding] CIRAD & ORSTOM, Montpellier, France. pp. 217—239.
Child, R., l974. Coconuts. 2nd edition. Longmans, London, United Kingdom. 335 pp.
Haas, A. & Wilson, L. (Editors), 1985. Coconut wood: processing and use. Food and Agriculture Organization (FAO), Rome, Italy. 58 pp.
Harries, H.C., 1995. Coconut (Cocos nucifera). In: Smartt, J. & Simmonds, N.W. (Editors): Evolution of crop plants. Longman, Scientific & Technical, Harlow, United Kingdom. pp. 389—394.
Maftei, M., 1998. Coconut in oils and fats market: trends and developments. Proceedings of the 35th Coconut Technical Panel of the Asian Pacific Coconut Community (COCOTECH) Meeting, July 1998, Bali, Indonesia. Asian and Pacific Coconut Community, Jakarta, Indonesia. pp. 1—25.
Magat, S.S., 1999. Coconut-based farming systems: technology notes for practitioners. Philippine Coconut Authority-Agricultural Research and Development Branch (ARD), Diliman, Quezon City, the Philippines. 97pp.
Magat, S.S., 1999. Production management of coconut (Cocos nucifera). Philippine Coconut Authority-ARD Diliman, Quezon City, the Philippines. 97 pp.
Menon, K.P.V. & Pandalai, K.M., 1958. The coconut palm, a monograph. Indian Central Coconut Committee, Ernakulam, India. 384 pp.
Ohler, J.G. (Editor), 1999. Modern coconut management, palm cultivation and products. Intermediate Technology Publications, London, United Kingdom. 458 pp.
Perry, L.M., 1980. Medicinal plants of East and South-East Asia: attributed properties and uses. MIT Press, Cambridge, Massachusetts, United States. p. 304.
Bourdeix, R., Baudouin, L., Billotte, N., Labouise, J.P. & Noiret, J.M., 1997. Le Cocotier [The Coconut]. In: Charrier, A., Jackot, M., Hamon, S. & Nicolas, D. (Editors): L'Amélioration des plants tropicales [Tropical plant breeding] CIRAD & ORSTOM, Montpellier, France. pp. 217—239.
Child, R., l974. Coconuts. 2nd edition. Longmans, London, United Kingdom. 335 pp.
Haas, A. & Wilson, L. (Editors), 1985. Coconut wood: processing and use. Food and Agriculture Organization (FAO), Rome, Italy. 58 pp.
Harries, H.C., 1995. Coconut (Cocos nucifera). In: Smartt, J. & Simmonds, N.W. (Editors): Evolution of crop plants. Longman, Scientific & Technical, Harlow, United Kingdom. pp. 389—394.
Maftei, M., 1998. Coconut in oils and fats market: trends and developments. Proceedings of the 35th Coconut Technical Panel of the Asian Pacific Coconut Community (COCOTECH) Meeting, July 1998, Bali, Indonesia. Asian and Pacific Coconut Community, Jakarta, Indonesia. pp. 1—25.
Magat, S.S., 1999. Coconut-based farming systems: technology notes for practitioners. Philippine Coconut Authority-Agricultural Research and Development Branch (ARD), Diliman, Quezon City, the Philippines. 97pp.
Magat, S.S., 1999. Production management of coconut (Cocos nucifera). Philippine Coconut Authority-ARD Diliman, Quezon City, the Philippines. 97 pp.
Menon, K.P.V. & Pandalai, K.M., 1958. The coconut palm, a monograph. Indian Central Coconut Committee, Ernakulam, India. 384 pp.
Ohler, J.G. (Editor), 1999. Modern coconut management, palm cultivation and products. Intermediate Technology Publications, London, United Kingdom. 458 pp.
Perry, L.M., 1980. Medicinal plants of East and South-East Asia: attributed properties and uses. MIT Press, Cambridge, Massachusetts, United States. p. 304.
Author(s)
J.G. Ohler & S.S. Magat
Correct Citation of this Article
Ohler, J.G. & Magat, S.S., 2001. Cocos nucifera L.. In: van der Vossen, H.A.M. and Umali, B.E. (Editors): Plant Resources of South-East Asia No 14: Vegetable oils and fats. PROSEA Foundation, Bogor, Indonesia. Database record: prota4u.org/prosea

All texts are licensed under a Creative Commons Attribution-Noncommercial-Share Alike 3.0 Netherlands License
This license does not include the illustrations (Maps,drawings,pictures); these remain all under copyright.